Acetylcholinesterase (abbreviated AChE , English AChE , EC 3.1.1.7 ) is a hydrolytic enzyme from the esterase family, which is contained in synapses and catalyzes the hydrolysis of the neurotransmitter acetylcholine to choline and the residue of acetic acid . The reaction catalyzed by acetylcholinesterase is necessary for the deactivation of acetylcholine in the synaptic cleft and the transition of the target cell to a resting state (for example, to relax muscle cells). Therefore , acetylcholinesterase inhibitors (organophosphorus insecticides , DFF , sarin , soman and V-gases , fasciculin and some other snake venom peptides ) are powerful toxins whose effects on the human body usually lead to death from convulsions of the respiratory muscles.
| Acetylcholinesterase | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering based on 1b41 | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbol | ; ACEE ARACHE; N-ACHE; Yt | ||||||||||||
| External IDs | ChEMBL : GeneCards : | ||||||||||||
| EC number | |||||||||||||
| |||||||||||||
| RNA expression profile | |||||||||||||
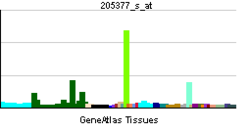 | |||||||||||||
 | |||||||||||||
| Orthologists | |||||||||||||
| View | Person | Mouse | |||||||||||
| Entrez | |||||||||||||
| Ensembl | |||||||||||||
| Uniprot | |||||||||||||
| RefSeq (mRNA) | |||||||||||||
| RefSeq (protein) | |||||||||||||
| Locus (UCSC) | |||||||||||||
| Search PubMed | |||||||||||||
The gene encoding the human acetylcholinesterase enzyme is located on the long arm of the seventh chromosome. [1] [2]
In humans, due to alternative splicing , four AChE isoforms are formed - T, H, R, and 4 [3] .
In synapses (in particular, in the terminal plates of neuromuscular synapses) AChE is present in the form of a tetramer of the T? Isoform attached to a collagen-like protein encoded by a separate COLQ gene [4] . Mutation of this gene is one of the most common causes of hereditary myasthenia gravis ( myasthenia gravis ) [5] . Using a collagen-like “tail”, acetylcholinesterase is attached to the proteoglycan perlecan , which is part of the synapse basal plate. Perlecan, in turn, attaches to the distroglycan complex integrated into the postsynaptic membrane of muscle cells [6] .
Acetylcholinesterase is found in the plasma membranes of erythrocytes (isoform H anchored in the membrane with attached fatty acid) [3] [4] and is a blood Yt antigen . [3] [4] [5] The His-353 variant (the usual variant with histidine at the 353 position of the chain) corresponds to the Yt (a) group, the rare Asn-353 variant corresponds to the Yt (b) group.
In neurons, AChE can be localized intracellularly (T isoform). Accumulation of AChE inside the nuclei of neuroblastoma cells has been shown to lead to apoptosis [6]
See also
- Cholinesterase
- Acetylcholinesterase inhibitors
- Acetylcholinesterase in the psychological encyclopedia (inaccessible link)
Notes
- ↑ Ehrlich G., Viegas-Pequignot E., Ginzberg D., et al. Mapping the human acetylcholinesterase gene to chromosome 7q22 by fluorescent in situ hybridization coupled with selective PCR amplification from a somatic hybrid cell panel and chromosome-sorted DNA libraries. (English) // Genomics : journal. - Academic Press , 1992. - Vol. 13 , no. 4 . - P. 1192-1197 . - DOI : 10.1016 / 0888-7543 (92) 90037-S . - PMID 1380483 .
- ↑ Getman DK, Eubanks JH, Camp S., et al. The human gene encoding acetylcholinesterase is located on the long arm of chromosome 7. (Eng.) // Am. J. Hum. Genet. : journal. - 1992. - Vol. 51 , no. 1 . - P. 170-177 . - PMID 1609795 .
- ↑ 1 2 G. Daniels. Functions of red cell surface proteins (Eng.) // Vox Sanguinis : journal. - 2007. - Vol. 93 . - P. 331-340 . - DOI : 10.1111 / j.1423-0410.2007.00970.x .
- ↑ 1 2 Spring FA, Gardner B., Anstee DJ Evidence that the antigens of the Yt blood group system are located on human erythrocyte acetylcholinesterase. (Eng.) // Blood : journal. - American Society of Hematology 1992. - Vol. 80 , no. 8 . - P. 2136-2141 . - PMID 1391965 .
- ↑ AChE (acetylcholinesterase, cholinesterase) .
- ↑ Yang L., He H. Y., Zhang X. J. Increased expression of intranuclear AChE involved in apoptosis of SK-N-SH cells. Neurosci. Res. 42: 261-268 (2002) [PubMed: 11985878].
Literature
- Silman I., Futerman AH Modes of attachment of acetylcholinesterase to the surface membrane. (English) // Eur. J. Biochem. : journal. - 1988. - Vol. 170 , no. 1-2 . - P. 11-22 . - DOI : 10.1111 / j.1432-1033.1987.tb13662.x . - PMID 3319614 .
- Soreq H., Seidman S. Acetylcholinesterase - new roles for an old actor. (Eng.) // Nat. Rev. Neurosci. : journal. - 2001. - Vol. 2 , no. 4 . - P. 294-302 . - DOI : 10.1038 / 35067589 . - PMID 11283752 .
- Shen T., Tai K., Henchman RH, McCammon JA Molecular dynamics of acetylcholinesterase. (English) // Accounts of Chemical Research : journal. - 2003. - Vol. 35 , no. 6 . - P. 332-340 . - DOI : 10.1021 / ar010025i . - PMID 12069617 .
- Pakaski M., Kasa P. Role of acetylcholinesterase inhibitors in the metabolism of amyloid precursor protein. (English) // Current drug targets. CNS and neurological disorders: journal. - 2003. - Vol. 2 , no. 3 . - P. 163-171 . - DOI : 10.2174 / 1568007033482869 . - PMID 12769797 .
- Meshorer E., Soreq H. Virtues and woes of AChE alternative splicing in stress-related neuropathologies. (Eng.) // Trends Neurosci. : journal. - 2006. - Vol. 29 , no. 4 . - P. 216-224 . - DOI : 10.1016 / j.tins.2006.02.005 . - PMID 16516310 .
- Shafferman A., Kronman C., Flashner Y., et al. Mutagenesis of human acetylcholinesterase. Identification of residues involved in catalytic activity and in polypeptide folding. (English) // J. Biol. Chem. : journal. - 1992. - Vol. 267 , no. 25 . - P. 17640-17648 . - PMID 1517212 .
- Li Y., Camp S., Rachinsky TL, et al. Gene structure of mammalian acetylcholinesterase. Alternative exons dictate tissue-specific expression. (English) // J. Biol. Chem. : journal. - 1992. - Vol. 266 , no. 34 . - P. 23083-23090 . - PMID 1744105 .
- Velan B., Grosfeld H., Kronman C., et al. The effect of elimination of intersubunit disulfide bonds on the activity, assembly, and secretion of recombinant human acetylcholinesterase. Expression of acetylcholinesterase Cys-580 ---- Ala mutant. (English) // J. Biol. Chem. : journal. - 1992. - Vol. 266 , no. 35 . - P. 23977-23984 . - PMID 1748670 .
- Soreq H., Ben-Aziz R., Prody CA, et al. Molecular cloning and construction of the coding region for human acetylcholinesterase reveals a G + C-rich attenuating structure. (Eng.) // Proceedings of the National Academy of Sciences of the United States of America : journal. - 1991. - Vol. 87 , no. 24 . - P. 9688-9692 . - DOI : 10.1073 / pnas.87.24.9688 . - PMID 2263619 .
- Chhajlani V., Derr D., Earles B., et al. Purification and partial amino acid sequence analysis of human erythrocyte acetylcholinesterase. (English) // FEBS Lett. : journal. - 1989. - Vol. 247 , no. 2 . - P. 279-282 . - DOI : 10.1016 / 0014-5793 (89) 81352-3 . - PMID 2714437 .
- Lapidot-Lifson Y., Prody CA, Ginzberg D., et al. Coamplification of human acetylcholinesterase and butyrylcholinesterase genes in blood cells: correlation with various leukemias and abnormal megakaryocytopoiesis. (Eng.) // Proceedings of the National Academy of Sciences of the United States of America : journal. - 1989. - Vol. 86 , no. 12 . - P. 4715-4719 . - DOI : 10.1073 / pnas.86.12.4715 . - PMID 2734315 .
- Bazelyansky M., Robey E., Kirsch JF Fractional diffusion-limited component of reactions catalyzed by acetylcholinesterase. (English) // Biochemistry: journal. - 1986. - Vol. 25 , no. 1 . - P. 125-130 . - DOI : 10.1021 / bi00349a019 . - PMID 3954986 .
- Gaston SM, Marchase RB, Jakoi ER Brain ligatin: a membrane lectin that binds acetylcholinesterase. (English) // J. Cell. Biochem. : journal. - 1982. - Vol. 18 , no. 4 . - P. 447-459 . - DOI : 10.1002 / jcb.1982.240180406 . - PMID 7085778 .
- Ordentlich A., Barak D., Kronman C., et al. Contribution of aromatic moieties of tyrosine 133 and of the anionic subsite tryptophan 86 to catalytic efficiency and allosteric modulation of acetylcholinesterase. (English) // J. Biol. Chem. : journal. - 1995. - Vol. 270 , no. 5 . - P. 2082-2091 . - DOI : 10.1074 / jbc.270.5.2082 . - PMID 7836436 .
- Maruyama K., Sugano S. Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides. (Eng.) // Gene : journal. - Elsevier , 1994. - Vol. 138 , no. 1-2 . - P. 171-174 . - DOI : 10.1016 / 0378-1119 (94) 90802-8 . - PMID 8125298 .
- Ben Aziz-Aloya R., Sternfeld M., Soreq H. Promoter elements and alternative splicing in the human ACHE gene. (English) // Prog. Brain Res. : journal. - 1994. - Vol. 98 . - P. 147-153 . - PMID 8248502 .
- Massoulie J., Pezzementi L., Bon S., Krejci E., Valette F. Molecular and Cellular Biology of Cholinesterases. (unspecified) // Prog. Brain Res .. - 1993.- T. 93 . - S. 31-91 . - PMID 8321908 .