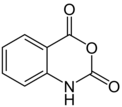
Isatoic anhydride is a chemical compound from the group of nitrogen-oxygen heterocycles with a keto group.
 | |
| General | |
|---|---|
Reg. CAS number | |
| Reg. EINECS number | 100.003.869 |
PubChem CID | |
CompTox Dashboard (EPA) | |
| The properties | |
Chemical formula | C 8 H 5 N O 3 |
| Molar mass | 163.132 gmol −1 |
| Appearance | odorless beige solid |
| Melting point | 243 ° C (469 ° F; 516 K) |
Data is provided for standard conditions (25 ° C, 100 kPa) , unless otherwise indicated. | |
Reactions
Hydrolysis gives carbon dioxide and anthranilic acid. Alcoholysis proceeds similarly, giving ether:
- C 6 H 4 C 2 O 3 NH + ROH → C 6 H 4 (CO 2 R) (NH 2 ) + CO 2
Amines also affect ring opening. Active compounds and methylene carbanions replace oxygen, giving hydroxyl quinolinone derivatives. Deprotonation followed by alkylation gives N-substituted derivatives. Sodium azide gives benzimidazolone via isocyanate. [1] Isatoic anhydride is used as a blowing agent in the polymer industry because it emits carbon dioxide .
Isatoic anhydride can be obtained by passing phosgene into a solution of anthranilic acid in aqueous hydrochloric acid [2] . This synthesis was first performed in 1899 by on the basis of discoveries in the field of synthesis of .
Properties
Isatoic anhydride is an odorless, combustible, moisture-sensitive, solid substance that is very slightly soluble in water. It decomposes on heating above 350 ° C. [3] .
Application
Citric anhydride is an intermediate product for various applications. It is used in coatings and serves as the starting material for agroactive ingredients. [4] .
Recommendations
- ↑ Coppola, Gary M. The Chemistry of Isatoic Anhydride (Neopr.) // Synthesis. - 1980 .-- T. 7 . - S. 505-36 . - DOI : 10.1055 / s-1980-29110 .
- ↑ Isatinic anhydride input in the database of hazardous substances
- ↑ Entrance to CAS No. 118-48-9 at GESTIS Bank for IFAs
- ↑ BASF Isatinic Anhydride