Vinblastine is a cytostatic agent , a drug from the group of vinca alkaloids ( lat. Vinca rosea L., Catharanthus roseus ).
| Vinblastine | |
|---|---|
| Vinblastine | |
 | |
| Chemical compound | |
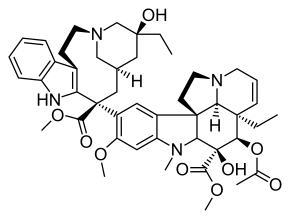
| IUPAC | (3aR, 4R, 5S, 5aR, 10bR, 13aR) -4-acetoxy-3a-ethyl-9 - [(5S, 7R, 9S) -5-ethyl-5-hydroxy-9-methoxycarbonyl-1,4,5 , 6,7,8,9,10-octahydro-2H-3,7-methano-azacycloundecyno [5,4-b] indol-9-yl] -5-hydroxy-8-methoxy-6-methyl- 3a, 4,5,5a, 6,11,12,13a-octahydro-1H-indolysino- [8,1-cd] |
| Gross formula | C 46 H 58 N 4 O 9 |
| Molar mass | 810.974 g / mol |
| Cas | |
| PubChem | |
| Drugbank | |
| Classification | |
| ATX | |
| Pharmacokinetics | |
| Bioavailable | n / a |
| Metabolism | Hepatic ( CYP3A4- linked) |
| The half-life. | 24.8 hours (terminal) |
| Excretion | With bile and kidneys |
| Dosage Forms | |
| lyophilisate for solution for intravenous administration | |
| Route of administration | |
| intravenously | |
| Other names | |
| Velbe, Vero-Vinblastine water, Vinblastin-LENS, Vinblastin-Richter, Vinblastin-Teva, Vinblastine sulfate | |
Content
Pharmacological action
The mechanism of action is associated with tubulin blockade and cell division arrest in metaphase.
Pharmacokinetics
To a small extent penetrates the BBB . Plasma protein binding is 75%. Biotransformed in the liver with the formation of active metabolites . It is mainly excreted with bile , partially by the kidneys .
Indications
Lymphogranulomatosis , non-Hodgkin’s lymphomas , germ cell tumors of the testis and ovaries, choriocarcinoma (resistant to other chemotherapeutic drugs), Kaposi’s sarcoma , fungoid mycosis (severe forms), Letterera-Sieve disease , kidney cancer, bladder cancer , neuroblastoma , cancer breast cancer .
Dosage
Installed individually, depending on the indications and stage of the disease, the state of the hematopoietic system, anti-tumor therapy regimen.
Side effect
From the central nervous system: neuropathy , peripheral nerve neuritis , headache , depression , cramps .
From the hemopoietic system: leukopenia , granulocytopenia , thrombocytopenia , anemia .
From the digestive system: anorexia , nausea , vomiting , abdominal pain, paralytic ileus , constipation , diarrhea , ulcerative stomatitis , hemorrhagic enterocolitis .
From the cardiovascular system: increased blood pressure , the development of myocardial infarction , cerebrovascular accident, increased symptoms of Raynaud's disease .
From the respiratory system: acute respiratory failure , bronchospasm .
From the reproductive system: azoospermia , amenorrhea .
Other: alopecia , bone pain.
Contraindications
Severe leukopenia , pregnancy , hypersensitivity to vinblastine.
Pregnancy and lactation
Vinblastine is contraindicated in pregnancy. If necessary, use during lactation should stop breastfeeding.
When used in women of childbearing age, it is recommended to use reliable methods of contraception .
In experimental studies, the teratogenic effect of vinblastine was established.
Special instructions
Vinblastine is used with caution in patients with chickenpox (including recently transferred or after contact with patients), herpes zoster , other acute infectious diseases, gout , nephrolithiasis (including a history ). In patients with impaired liver function, the risk of toxic effects of vinblastine is increased.
Use with caution during treatment with drugs that inhibit the activity of liver cytochrome P450 isoenzymes of the CYP3A system.
The maximum depression of hematopoiesis (primarily a decrease in the number of leukocytes in peripheral blood) is achieved after 5-10 days of using vinblastine. Normalization of the number of leukocytes in peripheral blood is observed 7-14 days after stopping the drug. The development of thrombocytopenia (less than 200,000 / μl) is most likely in patients who have received previous antitumor or radiation therapy . Normalization of the platelet count is noted, as a rule, a few days after the abolition of vinblastine.
The risk of developing leukopenia with vinblastine is increased in patients with cachexia and ulcerative lesions of the skin, therefore, patients with the above conditions are not recommended. In patients with bone marrow metastases , a marked decrease in the number of leukocytes and platelets was noted after the use of vinblastine in medium doses. In these cases, further use of vinblastine is not indicated.
During therapy, the activity of liver transaminases and LDH , the level of bilirubin and the concentration of uric acid in the blood plasma should be monitored.
During the treatment period, vaccination of patients and their families is not recommended.
Intra-shell administration of vinblastine can be fatal. In case of accidental contact with the eyes, severe inflammation may occur.
Vinblastine in the form of lyophilized powder for injection is included in the List of Essential Drugs .
Drug Interactions
With the simultaneous use of liver cytochrome P450 system with inhibitors of the activity of CYP3A isoenzymes, an earlier appearance and / or aggravation of the severity of side effects of vinblastine is possible. With simultaneous use with vinblastine, a decrease in the concentration of phenytoin in blood plasma and a decrease in its anticonvulsant activity is possible, apparently due to a decrease in absorption , an increase in the rate of metabolism and elimination of phenytoin.
With the simultaneous use of vinblastine in high doses with interferon alpha-n1, severe myelode depression is possible.