Tetraethyl pyrophosphate , tetraethyl diphosphate , and also tetraethyl ether of pyrophosphoric acid (abbr. TEPP ) - organophosphorus compound , ester , extremely toxic substance, has an unusually strong contact insecticidal effect, belongs to the group of tetraalkyl diphosphates. The toxicity of TEPP, like many other FOS, is caused by irreversible inhibition of the enzyme acetylcholinesterase , as well as some serine proteases ( chymotrypsin and others), in extremely low concentrations (10 -5-10 -8-8 M or less), causing pathophysiological effects from the central nervous system (convulsions , kinetoses , cramps, paralysis, impaired consciousness, coma), cardiovascular (bradycardia, a sharp drop in blood pressure, collapse), digestive systems (salivation, vomiting, dyspeptic symptoms, etc.), respiratory (bronchospasm), and as a result of summer ln outcome. Due to these negative properties, it has a ban on production and use in Russia.
| Tetraethyl pyrophosphate | |
|---|---|
 | |
 | |
| Are common | |
| Systematic name | Tetraethyl diphosphate |
| Abbreviations | TEPF |
| Traditional names | Tetraethyl pyrophosphate |
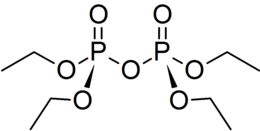
| Chem. formula | C 8 H 20 O 7 P 2 |
| Rat formula | [C 2 H 5 O] 4 P 2 O 3 |
| Physical properties | |
| condition | colorless hygroscopic liquid |
| Molar mass | 290.19 g / mol |
| Density | 1.189 g / cm³ |
| Thermal properties | |
| T. melt. | 0 ° C |
| T. bale. | 124 ° C |
| Steam pressure | |
| Optical properties | |
| Refractive index | 1.4071 |
| Classification | |
| Reg. CAS number | 107-49-3 |
| PubChem | |
| Reg. EINECS number | |
| Smiles | |
| Inchi | |
| RTECS | |
| Chebi | |
| UN number | |
| ChemSpider | |
| Security | |
| LD 50 | 0.5 mg / kg (rat, oral) |
| Toxicity | Extremely toxic, the strongest poison is a nerve agent.  |
Content
Physico-chemical properties
It is a colorless or slightly yellowish (technical preparation), slightly mobile liquid, with a pleasant odor, readily soluble in water, also soluble in organic solvents: in benzene , toluene , carbon tetrachloride , acetone , ethanol , poorly soluble in naphtha and petroleum ether . Due to the high hygroscopicity of TEPP, it is an unstable compound and quickly hydrolyzes (~ 7 h at 25 ° С) to low toxic products (diethylphosphoric acid). When heated above 170 ° C, TEPP decomposes, and at a temperature above 208 ° C there is a rapid evolution of ethylene [2] .
Getting
TEPP was one of the first synthesized FOS. The synthesis was first carried out by Moshnin in 1850, in the laboratory of S. Wurz (Paris). In 1854, F. Clermont synthesized ether from the silver salt of pyrophosphoric acid and ethyl iodide according to the scheme [3] :
.
For the first time in the USSR, the synthesis of TEPP was carried out at the chemical institute of the Kazan branch of the USSR Academy of Sciences A.E. Arbuzov in 1931.
Application
Due to the extremely high toxicity of TEPP, its use is limited; at present, it is used as insecticidal mixtures with other FOS. TEPF cannot be used as a chemical warfare agent due to its low stability and rapid hydrolysis (it is practically ineffective in a humid climate).
Effectiveness as a Pesticide
TEPP is one of the first organophosphorus insecticides and acaricides with an extremely strong contact effect (even at negligible concentrations - 0.02% or less caused complete death of aphids and ticks), more severe than nicotine . Despite this, TEPP does not have a systemic effect, that is, it does not spread throughout the body of the insect. TEPF can be used as an effective fumigant (the drug is in the form of an aerosol). Plants sprayed with TEPF become safe in a day, since it is completely hydrolyzed.
A technical preparation containing up to 40% TEPP is called bladan. First used as an insecticide in 1943 in Germany.
Synonyms and trade names
- Bladan (product of Farbenfabrike Bayer, Germany),
- Vapoton (California spray-chemical Co, USA),
- Killax
- Mortopal,
- Nifos T ( Monsanto , United States),
- Tetron (American Potash and Chemical Company, USA).
Toxicology and Safety
TEPP is a typical representative of organophosphorus compounds with a pronounced anticholinesterase effect.
Molecular Mechanism of Action of TEPF
The action of TEPP molecules on the active center of cholinesterase , like all serine proteases, is based on the binding of serine and the formation of covalently strong teraethylserine pyrophosphate with it, which is very slightly hydrolyzed, thereby changing the conformation of the enzyme, making it inactive. Inactivation of TEPF serine proteases is observed even at extremely low concentrations.
Toxicity
TEPF is an extremely toxic substance. LD50 are given in the table.
| Organism | LD50 in mg / kg |
|---|---|
| Rats | 0.5-1.7 (oral) |
| Mice | 5-7 (intraperitoneal) |
| Rabbits | 6-7 (cutaneous) |
| Cats | 1.2-3 (cutaneous) |
| Person | 0.7-1 (oral) <0.4 (aerosol) |
The lethal dose for humans with oral administration of TEPP is about 80-100 mg. The greatest toxic effect develops when inhaled vapors or aerosol TEPF. LD100 with a respiratory route of penetration is reduced to 15-30 mg.
The main ways of penetration of TEPF into the human body are respiratory (respiratory), oral or contact (cutaneous). Signs of poisoning can occur within minutes. Signs of poisoning include a sharp increase in perspiration, miosis (narrowing of the pupils), salivation, dizziness, decreased muscle activity ( myasthenia gravis ), hypotension, bradycardia , visual impairment, cramps , dyspeptic symptoms, nausea , vomiting , diarrhea, bronchospasm in case of inhalation of an aerosol or vapor. At high doses (40-70 mg), an almost instantaneous damage to the central nervous system occurs, loss of consciousness, convulsions or paralysis , as a result of coma, can lead to death within a few hours.
Atropine and 2-PAM ( pralidoxime ) are effective antidotes for TEPF poisoning.
Security
TEPF refers to substances with a very high hazard (hazard class I). In Russia, this substance is prohibited in production and use. In the USA, the MPC is 0.05 mg / m 3 .
Notes
- ↑ http://www.cdc.gov/niosh/npg/npgd0590.html
- ↑ Schrader G. New organophosphorus insecticides / Translation from German A. G. Zenkevich, Ph.D. Chem. Sciences Y. A. Mandelvaum, Ph.D. Chem. Sciences K. D. Shvetsovoj-Shilovskoy, Edited by doctor chem. Mauk, prof. N. N. Melnikova. - 2nd ed. - M .: WORLD, 1965.
- ↑ Fest, Christa. The Chemistry of Organophosphorus Pesticides - Springer / Christa Fest, Karl-Julius Schmidt. - DOI : 10.1007 / 978-3-642-68441-8 .