Dabigatran , sold under the brand name Pradaxa in particular, is an anticoagulant that can be used orally. It is used for different cases and in some cases it is an alternative to warfarin ,
| Dabigatran etexilate | |
|---|---|
 | |
| Chemical compound | |
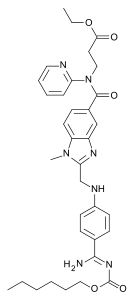
| IUPAC | Ethyl N - [(2 - {[(4- { N ́ - [(hexyloxy) carbonyl] carbamimidoyl} phenyl) amino] methyl} -1-methyl-1 H- benzimidazol-5-yl) carbonyl] - N -2 -pyridinyl-β-alaninate |
| Gross formula | C 25 H 25 N 7 O 3 |
| Molar mass | 627.734 g / mol |
| Cas | |
| PubChem | |
| Drugbank | |
| Classification | |
| ATX | |
| Pharmacokinetics | |
| Bioavailable | 3–7% [1] |
| Plasma Protein Binding | 35% [1] |
| The half-life. | 12–17 hours [1] |
| Route of administration | |
| By mouth | |
In case of severe bleeding, he has an antidote, idarucizumab. A study sponsored by the manufacturer showed that Idarucizumab is effective in neutralizing dabigatran in a few minutes. [2]
It is a direct thrombin inhibitor. It was developed by the Beringer Ingelheim pharmaceutical company.
Content
- 1 Medical use
- 2 Contraindications
- 3 side effects
- 4 Pharmacokinetics
- 5 History
- 6 Research
- 7 References
Medical Use
Dabigatran is used to prevent strokes in patients with atrial fibrillation not caused by heart valve problems, as well as deep vein thrombosis and pulmonary embolism in individuals who have been treated for 5-10 days with a parenteral anticoagulant (usually low molecular weight heparin ), and to prevent deep vein thrombosis and pulmonary embolism in some cases. [3]
It is as effective as warfarin in preventing non-hemorrhagic strokes and embolic events in patients with atrial fibrillation not due to valve problems. [four]
Contraindications
Dabigatran is contraindicated in patients who have active pathological bleeding, since dabigatran can increase the risk of bleeding, and can also cause serious and potentially life-threatening bleeding. [5] Dabigatran is also contraindicated in patients who have a history of serious hypersensitivity reactions to dabigatran (eg, anaphylaxis or anaphylactic shock). the use of dabigatran should also be avoided in patients with mechanical prosthetic heart valves due to the increased risk of thromboembolic events (e.g., valve, thrombosis, stroke and myocardial infarction ) and massive bleeding associated with dabigatran in this population. [6] [7]
Side Effects
The most commonly reported side effect of dabigatran is an upset gastrointestinal tract. Compared to people using warfarin, patients taking dabigatran had less life-threatening bleeding, less often small and large bleeding, including intracranial bleeding, but the frequency of gastrointestinal bleeding was significantly higher. Dabigatran capsules contain tartaric acid, which lowers the pH of the stomach and is necessary for proper absorption. Low pH has previously been associated with dyspepsia ; some suggest that this plays a role in the increased risk of gastrointestinal bleeding. [8]
The risk of myocardial infarction (heart attacks) is increased. [9]
Pharmacokinetics
Dabigatran has a half-life of about 12-14 hours, has a maximum effect within 2-3 hours after administration. [10] Fatty foods delay the absorption of dabigatran, although the bioavailability of the drug does not change. one study showed that absorption can be moderately reduced when taken with proton pump inhibitors. [11] The excretion of the drug with P-glycoprotein slows down in patients taking strong inhibitors such as quinidine , verapamil and amiodarone , which increases plasma levels of dabigatran. [12]
History
Dabigatran (BIBR compounds 953) was found in a group of chemicals with a similar structure in the benzamidine base of thrombin, an α-NAPAP inhibitor ( H- Alpha- (2-naphthylsulfonylglycyl) -4-amidinophenylalanine piperidide), which has been known since the 1980s as a powerful inhibitor of various serine proteases , in particular thrombin, as well as trypsin . The addition of ethyl ester and hexylocarbamide hydrophobic side chains led to the bioavailability of the prodrug , BIBR 1048 (dabigatran etexilate). [13]
On March 18, 2008, the European Medicines Agency issued a registration certificate for Etexilate for the prevention of thromboembolic diseases after endoprosthetics of the hip or knee joint and for non-valve atrial fibrillation. [fourteen]
The UK National Health Service has approved the use of dabigatran for the prevention of blood clots in the femur and knee joints of patients. According to a BBC article in 2008, Dabigatran is expected to cost nhs £ 4.20 a day, which looked like several other anticoagulants . [fifteen]
Initially, there was no definite way to reverse the anticoagulant effect of dabigatran in case of major bleeding. [16] [17] unlike warfarin, [18] the antidote dabigatran idarucizumab was approved by the FDA in 2015. [19]
Etexilate received a Compliance Notice (NLC) from the Department of Health Canada on June 10, 2008, [20] for the prevention of blood clots in patients undergoing hip replacement or a complete knee replacement surgery. Approvals for patients with atrial fibrillation risk of stroke came in October 2010. [21] [22]
In the US, the Food and Drug Administration (FDA) approved Etexilate on October 19, 2010, for the prevention of stroke in patients with non-valve atrial fibrillation. [23] [24] [25] [26] , albeit with some limitations. [27]
. [28]
In May 2014, the Office announced the results of a large study of dabigatran versus warfarin in 134,000 patients. The agency concluded that dabigatran is associated with a lower risk of overall mortality, ischemic stroke, and cerebral hemorrhage than warfarin. Gastrointestinal bleeding was more common in patients receiving dabigatran than in patients receiving warfarin. The risk of a heart attack was similar between the two drugs. The agency reiterated its view that dabigatran is generally beneficial. [29]
On July 26, 2014, British Medical Journal (bmj's) published a series of investigations that accused Beringer of hiding important information about the need for monitoring to protect patients from severe bleeding, especially in older people. [30] [31]
Research
In August 2015, a study showed that the drug Idarucizumab can block the anticoagulant effect of dabigatran for several minutes. [32] Idarucizumab was approved in October 2015.
Links
- ↑ 1 2 3 Pradaxa Full Prescribing Information Archived on August 10, 2015. . Boehringer Ingelheim . October 2010.
- ↑ Pollack, Charles V .; Reilly, Paul A .; Eikelboom, John; Glund, Stephan; Verhamme, Peter; Bernstein, Richard A .; Dubiel, Robert; Huisman, Menno V .; Hylek, Elaine M. Idarucizumab for Dabigatran Reversal (Eng.) // The New England Journal of Medicine : journal. - 2015 .-- 6 August ( vol. 373 , no. 6 ). - P. 511-520 . - ISSN 1533-4406 . - DOI : 10.1056 / NEJMoa1502000 . - PMID 26095746 .
- ↑ http://www.drugs.com/pro/pradaxa.html Pradaxa
- ↑ Gómez-Outes, A. Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups (Eng.) // Thrombosis: journal. - 2013 .-- Vol. 2013 . - P. 640723 . - DOI : 10.1155 / 2013/640723 . - PMID 24455237 .
- ↑ Pradaxa (dabigatran etexilate mesylate) Prescribing Information: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ba74e3cd-b06f-4145-b284-5fd6b84ff3c9#Section_5.4 , accessed October 29, 2014.
- ↑ FDA Drug Safety Communication: Pradaxa (dabigatran etexilate mesylate) should not be used in patients with mechanical prosthetic heart valves . US Food and Drug Administration (FDA) . Date of treatment October 29, 2014.
- ↑ Eikelboom, JW Dabigatran versus Warfarin in Patients with Mechanical Heart Valves (Eng.) // N Engl J Med : journal. - 2013 .-- September ( vol. 369 ). - P. 1206-1214 . - DOI : 10.1056 / NEJMoa1300615 . - PMID 23991661 .
- ↑ ML Blommel; ML Blommel Dabigatran etexilate: A novel oral direct thrombin inhibitor (Eng.) // Am J Health Syst Pharm : journal. - 2011. - Vol. 68 , no. 16 . - P. 1506-1519 . - DOI : 10.2146 / ajhp100348 . - PMID 21817082 .
- ↑ Uchino K; Uchino K. Dabigatran associated with higher risk of acute coronary events - meta-analysis of noninferiority randomized controlled trials (Eng.) // Arch. Intern. Med. : journal. - 2012. - Vol. 172 , no. 5 . - P. 397-402 . - DOI : 10.1001 / archinternmed.2011.1666 . - PMID 22231617 . Archived April 23, 2012. Archived April 23, 2012 on Wayback Machine
- ↑ Chongnarungsin D; Chongnarungsin D. In-Depth Review of Stroke Prevention in Patients with Non-Valvular Atrial Fibrillation (Eng.) // Am. Med. J.: journal. - 2012. - Vol. 3 , no. 2 . - P. 100-103 . - DOI : 10.3844 / amjsp.2012.100.103 .
- ↑ Stangier J; Stangier J. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement (Eng.) // J Clin Pharmacol : journal. - 2005 .-- May ( vol. 45 , no. 5 ). - P. 555-563 . - DOI : 10.1177 / 0091270005274550 . - PMID 15831779 .
- ↑ Pradaxa Summary of Product Characteristics Archived March 22, 2011. .
- ↑ Hauel NH; Hauel NH Structure-based design of novel potent nonpeptide thrombin inhibitors (Eng.) // J Med Chem : journal. - 2002 .-- April ( vol. 45 , no. 9 ). - P. 1757-1766 . - DOI : 10.1021 / jm0109513 . - PMID 11960487 .
- ↑ Pradaxa EPAR unopened (inaccessible link) . European Medicines Agency . Date of treatment January 30, 2011. Archived on August 2, 2012.
- ↑ Clot drug 'could save thousands' , BBC News Online (April 20, 2008). Date of treatment April 21, 2008.
- ↑ Eerenberg, ES Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects (English) // Circulation : journal. - Lippincott Williams & Wilkins 2011 .-- 4 October ( vol. 124 , no. 14 ). - P. 1573-1579 . - DOI : 10.1161 / CIRCULATIONAHA.111.029017 . - PMID 21900088 .
- ↑ van Ryn J; van Ryn J. Dabigatran etexilate - a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity (Eng.) // Thrombosis and Haemostasis : journal. - 2010 .-- June ( vol. 103 , no. 6 ). - P. 1116-1127 . - DOI : 10.1160 / TH09-11-0758 . - PMID 20352166 .
- ↑ Hanley, JP Warfarin reversal (English) // Journal of Clinical Pathology : journal. - 2004 .-- November ( vol. 57 , no. 11 ). - P. 1132-1139 . - DOI : 10.1136 / jcp.2003.008904 . - PMID 15509671 .
- ↑ Boehringer Ingelheim (2014-06-26). Boehringer Ingelheim's Investigational Antidote for Pradaxa® (dabigatran etexilate mesylate) Receives FDA Breakthrough Therapy Designation . Press release . Retrieved 2014-07-26 .
- ↑ "Summary Basis of Decision (SBD): Pradax" Health Canada . 2008-11-06.
- ↑ Kirkey, Sharon . Approval of new drug heralds 'momentous' advance in stroke prevention , Montreal Gazette (October 29, 2010). Date of treatment October 29, 2010.
- ↑ "Pradax (Dabigatran Etexilate) Gains Approval In Canada For Stroke Prevention In Atrial Fibrillation" Medical News Today. October 28, 2010.
- ↑ Connolly, SJ Dabigatran versus warfarin in patients with atrial fibrillation // The New England Journal of Medicine | N Engl J Med : journal. - 2009 .-- September ( vol. 361 , no. 12 ). - P. 1139-1151 . - DOI : 10.1056 / NEJMoa0905561 . - PMID 19717844 .
- ↑ Turpie AG; Turpie AG New oral anticoagulants in atrial fibrillation (neopr.) // Eur Heart J . - 2008. - January ( t. 29 , No. 2 ). - S. 155-165 . - DOI : 10.1093 / eurheartj / ehm575 . - PMID 18096568 .
- ↑ Boehringer wins first US OK in blood-thinner race , Thomson Reuters (October 19, 2010). Date of treatment October 20, 2010.
- ↑ FDA approves Pradaxa to prevent stroke in people with atrial fibrillation , US Food and Drug Administration (FDA) (October 19, 2010).
- ↑ Merli G; Merli G. Use of emerging oral anticoagulants in clinical practice: translating results from clinical trials to orthopedic and general surgical patient populations (Eng.) // Ann Surg : journal. - 2009 .-- August ( vol. 250 , no. 2 ). - P. 219-228 . - DOI : 10.1097 / SLA.0b013e3181ae6dbe . - PMID 19638915 .
- ↑ Wann LS; Wann LS 2011 ACCF / AHA / HRS Focused Update on the Management of Patients With Atrial Fibrillation (Update on Dabigatran): A Report of the American College of Cardiology Foundation / American Heart Association Task Force on Practice Guidelines // Circulation : journal. - Lippincott Williams & Wilkins 2011 .-- March ( vol. 123 , no. 10 ). - P. 1144-1150 . - DOI : 10.1161 / CIR.0b013e31820f14c0 . - PMID 21321155 .
- ↑ FDA Drug Safety Communication: FDA study of Medicare patients finds risks lower for stroke and death but higher for gastrointestinal bleeding with Pradaxa (dabigatran) compared to warfarin .
- ↑ Cohen, D; Cohen, D. Dabigatran: how the drug company withheld important analyses (English) // BMJ : journal. - 2014 .-- July ( vol. 349 ). - P. g4670 . - DOI : 10.1136 / bmj.g4670 . - PMID 25055829 .
- ↑ Moore TJ; Moore TJ Dabigatran, bleeding, and the regulators (English) // BMJ : journal. - 2014 .-- July ( vol. 349 ). - P. g4517 . - DOI : 10.1136 / bmj.g4517 . - PMID 25056265 .
- ↑ Pollack, Charles V. Idarucizumab for Dabigatran Reversal (Eng.) // New England Journal of Medicine : journal. - 2015 .-- 1 January ( vol. 373 , no. 6 ). - P. 511-520 . - DOI : 10.1056 / nejmoa1502000 . - PMID 26095746 .