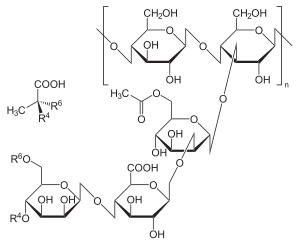
Xanthan gum ( Xanthan ) - a natural chemical compound ( C 35 H 49 O 29 ) n , food supplement E415, belongs to the group of stabilizers.
By its chemical nature, xanthan gum is a polysaccharide obtained by fermentation using the bacteria Xanthomonas campestris . In the life cycle of bacteria, it serves as protection against viruses and drying out, so it can be used in cosmetics to moisturize the skin. The production of xanthan is based on aerobic fermentation in an aqueous solution of carbohydrates , a source of nitrogen , etc., after which the medium is pasteurized and precipitated with alcohol or purified by microfiltration .
The properties of xanthan are regulated by changing the living conditions of bacteria. The polymer backbone is identical to the cellulose molecule. Branches are residues of glucose , mannose , glucuronic acid molecules as well as pyruvic acid (pyruvate) and acetyl groups. The number of pyruvate groups determines the viscosity of xanthan aqueous solutions. For food purposes, acid groups are neutralized by converting xanthan to potassium , sodium or calcium salts .
Application
Xanthan gum is used in food systems as thickeners , gelling agents and stabilizers . It is soluble in cold and hot water, milk, as well as in salt and sugar solutions. Xanthan molecules adsorb water to form a three-dimensional network of xanthan double helixes, which is similar in structure to a gel but has a lower viscosity . In this regard, xanthan is usually used as a thickener or stabilizer, and not a gelling agent.
The use of xanthan gum allows you to:
- increase the viscosity and elasticity of minced meat ;
- get a more stable and plastic structure of the finished product;
- reduce moisture loss during heat treatment and subsequent storage of finished products.
Xanthan solution is resistant to enzymes , alcohols , surfactants , acids (except hydrochloric ) and alkalis , high (up to 120ºС) and low (up to −18ºС) temperatures. In a mixture with other gums, the thickening effect is higher than for each thickener separately. Xanthan solution is characterized by high viscosity in the pH range from 2 to 12 and pseudoplasticity . Thanks to these properties, it forms a good structure, stabilizes products for a long time and lengthens their shelf life. It is widely used in the production of sauces , dairy products, ice cream , desserts , bakery products, drinks , etc.
In addition to the food and cosmetic industries, xanthan gum is used in oil and gas production processes. Xanthan gum is used as a builder in water-based drilling fluids , both fresh and highly mineralized. Xanthan gum has properties that are desirable for the solutions used in pumping and well workover. Xanthan gum is not a material for regulating filtration, however, it is well combined with filtration reducers such as CMC [1] Xanthan gum is used in concentrations from 0.6 to 6 kg / m 3 . [2]
See also
- Nutritional supplements
- E400-E499: Group of stabilizers
Notes
- ↑ A.L. Neverov *, A.V. Gusev, A.V. Mineev, V.P. Rozhkov. [ http://elib.sfu-kras.ru/bitstream/handle/2311/9652/11_Neverev.pdf?sequence=1&isAllowed=y Drilling fluids with a low solids content for drilling with SSC complexes based on Tagan bentonite deposits] (Rus. ) // Journal of Siberian Federal University. Engineering & Technologies 1 (2013 6) 95-106: Journal. - 2013 .-- S. 95-106 .
- ↑ Gray J.R., Darley G.S.G. Composition and properties of drilling agents (flushing fluids). - "The bowels", 1985. - S. 470. - 509 p.