The Passerini reaction is a three-component reaction between a carboxylic acid , a carbonyl compound ( aldehyde or ketone ) and isocyanide, the product of which is α-hydroxycarboxamide [1] [2] [3] .
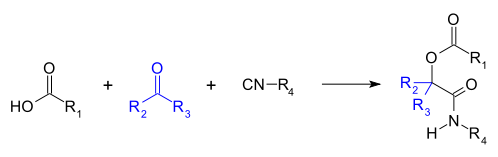
The reaction was discovered by Italian Mario Passerini from Florence in 1921. This was the first multicomponent reaction involving isocyanide; Now this reaction plays a central role in combinatorial chemistry [4] .
Recently, Denmark et al. (Denmark et al. ) Developed an enantheoselective catalyst for asymmetric Passerini reactions [5] .
Content
- 1 Reaction mechanism
- 1.1 Ionic mechanism
- 1.2 Agreed Mechanism
- 2 Application
- 3 See also
- 4 notes
Reaction Mechanism
Two possible reaction mechanisms have been put forward.
Ion Mechanism
In polar solvents such as methanol or water , the reaction occurs through protonation of the carbonyl group and subsequent nucleophilic addition of the isocyanide to form the protonated nitrile ion 3 . The addition of a carboxylate anion gives intermediate 4 . The transfer of the acyl moiety and the amide tautomerization leads to the formation of the desired ester 5 .
Agreed Mechanism
In nonpolar solvents and at high concentrations, the reaction probably proceeds according to an agreed mechanism [6] :
This mechanism consists of a trimolecular reaction between isocyanide (R-NC), a carboxylic acid and a carbonyl compound in a series of nucleophilic additions . The transition state of TS # is described as a five-membered ring with a partial covalent or double bond. The second step of the Passerini reaction is the transfer of the acyl group to a nearby hydroxyl group. There are facts in favor of such a mechanism: the reaction proceeds in a relatively nonpolar solvent (in accordance with the transition state) and the kinetics of the reaction depends on the concentration of all three reagents. This reaction serves as a good example of convergent synthesis .
Application
The Passerini reaction is used in many multicomponent reactions, for example, it occurs immediately after the Horner – Wodsworth – Emmons reaction in the synthesis of depsipeptide [7] :
Passerini multicomponent reactions have found application in the synthesis of polymers from renewable materials [8] .
See also
Notes
- ↑ Passerini, M .; Simone, L. Gazz. Chim. Ital. 1921 , 51 , 126-29.
- ↑ Passerini, M .; Ragni, G. Gazz. Chim. Ital. 1931 , 61 , 964-69.
- ↑ Riva, R .; Banfi, L. The Passerini Reaction (Neopr.) // Org. React .. - 2005 .-- T. 65 . - S. 1-140 . - DOI : 10.1002 / 0471264180.or065.01 . .
- ↑ Dömling, A .; Ugi, I. Angew. Chem. Int. Ed. Engl. 2000 , 39 , 3168-3210. (Review)
- ↑ Denmark, SE; Fan, Y. J. Org. Chem. 2005 , 70 , 9667-76. DOI : 10.1021 / jo050549m
- ↑ The Passirini Reaction L. Banfi, R. Riva in Organic Reactions vol. 65 LE Overman Ed. Wiley 2005 ISBN 0-471-68260-8
- ↑ A Flexible Six-Component Reaction To Access Constrained Depsipeptides Based on a Dihydropyridinone Core Monica Paravidino, Rachel Scheffelaar, Rob F. Schmitz, Frans JJ de Kanter, Marinus B. Groen, Eelco Ruijter, and Romano VA Orru J. Org. Chem. 2007 , 72, 10239-42 DOI : 10.1021 / jo701978v
- ↑ Kreye, O .; Tóth, T .; Meier, M. J. Am. Chem. Soc. , 2011, 133 (6), pp 1790-1792 [1] DOI : 10.1021 / ja1113003