Tranexamic acid ( TCA ) is a drug used to treat or prevent excessive blood loss from injury, surgery, hemophilia, heavy menstrual bleeding [1] . It is used in oral and intravenous forms [1] .
| Tranexamic acid | |
|---|---|
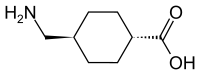 | |
 | |
| Chemical compound | |
| IUPAC | trans- 4- (aminomethyl) cyclohexanecarboxylic acid |
| Gross formula | C 8 H 15 NO 2 |
| Molar mass | 157.21 g / mol |
| CAS | |
| Pubchem | |
| Drugbank | |
| Classification | |
| ATH | |
| Pharmacokinetics | |
| Bioavailable | 34% |
| The period is half out. | 3.1 h |
| Mode of administration | |
| Injections and by mouth | |
Side effects are rare and include gastrointestinal effects, dizziness, fatigue, headache, hypersensitivity reactions [2] . this drug should be used with caution in people with kidney disease and those people who are at high risk of blood clots [1] . Tranexamic acid is safe for use in pregnant women. However, it should be used with caution in lactating women [1] .
Tranexamic acid is a synthetic analogue of the amino acid lysine .
Side Effects
Common side effects include [2] :
- Headaches (50.4 - 60.4%);
- Pain in the back (20.7 - 31.4%);
- Nasal sinus problem (25.4%);
- Abdominal pain (12–19.8%);
- Diarrhea (12.2%);
- Fatigue (5.2%);
- Anemia (5.6%).
Rare side effects include [2] :
- Pulmonary embolus ;
- Deep vein thrombosis ;
- Anaphylaxis ;
- Visual impairment.
Society and Culture
TXA has been added to the WHO list of essential drugs [3] . txa is inexpensive and treatment will be considered cost effective with high, medium and low income [4] .
Brand Names
Tranexamic acid is sold in the USA and Australia in tablet form as Lysteda and in intravenous infusion as Cyklokapron and Transamin, in the UK as Cyclo-F and Femstrual, in Asia as Transcam, as Traxyl in Bangladesh, in India, as Pause, in South America as Espercil, in Japan as Nicolda, in France and Romania as Exacyl, and in Egypt as Kapron. In the Philippines, her capsules are positioned as Hemostan and in Israel as Hexakapron .
Notes
- ↑ 1 2 3 4 Cyklokapron (tranexamic acid) Product Information . Circulation date November 3, 2015. Archived February 29, 2016.
- ↑ 1 2 3 Lysteda (tranexamic acid) Package Insert . accessdata.FDA.gov . The appeal date is November 2, 2015.
- ↑ 19th WHO Model List of Essential Medicines (April 2015) . WHO (April 2015). The appeal date is May 10, 2015.
- ↑ Guerriero, Carla; Cairns, John; Perel, Pablo; Shakur, Haleema; Roberts, Ian; Crash 2 Trial, Collaborators. Cost-Effectiveness Analysis of Administering Tranexamic Acid to Bleeding Trauma Patients Using Evidence from CRASH-2 Trial (Eng.) // PLoS ONE : journal. - 2011. - Vol. 6 , no. 5 - P. e18987 . - DOI : 10.1371 / journal.pone.0018987 . - . - PMID 21559279 .