N- Chlorosuccinimide is an organic compound, a chlorine derivative of succinimide . Used in organic synthesis in the reactions of electrophilic chlorination of sulphides , sulfoxides and ketones , as well as to obtain N- chloramines [1] .
N-Chlorosuccinimide | |
|---|---|
 | |
Are common | |
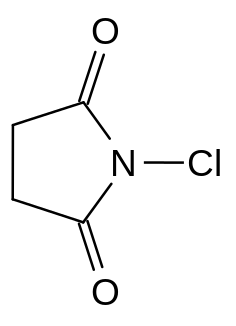
| Systematic name | 1-chloro-2,5-pyrrolidinedione |
| Abbreviations | NCS |
| Chem. formula | C₄H₄ClNO₂ |
Thermal properties | |
| T. melt. | 144–146 ℃ |
Classification | |
| CAS number | |
| Pubchem | |
| Chemspider | |
| EINECS number | 204-878-8 |
| CHEBI | 53203 |
SMILES | |
C1CC (= O) N (C1 = O) Cl | |
Inchi | |
1S / C4H4ClNO2 / c5-6-3 (7) 1-2-4 (6) 8 / h1-2H2 | |
| Data are given for standard conditions (25 ℃, 100 kPa) , unless otherwise specified. | |
Content
Physical Properties
The compound is a white crystal with a slight odor of chlorine . Soluble in water, slightly soluble in carbon tetrachloride , benzene , toluene and acetic acid , insoluble in ether [1] .
Application
Among all chlorinating agents, N- chlorosuccinimide is distinguished by relative ease of use and mild conditions under which chlorination occurs. One of the applications of this reagent is α-chlorination of carbonyl compounds . It can act directly on lithium enolates or silyl ethers , which makes it possible to chlorinate carbonyl compounds regioselectively , provided that enolate or silyl ether are also obtained with the necessary regioselectivity [1] .
N- chlorosuccinimide also chlorinates sulphides and sulfoxides in α-position. The resulting products are usually easily hydrolyzed, but serve as useful intermediates in the synthesis of other products. For example, this feature is used to convert benzyl bromides to benzaldehydes by replacing bromine with a phenylsulfide group, followed by α-chlorination and hydrolysis [1] .
N- chlorosuccinimide can chlorinate some aromatic compounds, for example, pyrroles and indoles , although the chlorination reaction is not as simple as the corresponding bromination and iodination reactions using N- bromosuccinimide and N- iodosuccinimide [1] .
It is convenient to convert secondary amines into chloramines using N- chlorosuccinimide, which provides a more convenient product isolation than an aqueous solution of hypochlorite [1] .
Cleaning
At long storage the reagent turns yellow and gets a strong smell of chlorine. You can estimate the purity using iodometry . Purification of N- chlorosuccinimide is carried out by crystallization from acetic acid [1] .
Precautions
N- chlorosuccinimide must be stored outside the access of atmospheric moisture and during cooling. It has an acute irritant and toxic effect similar to that of free halogens . It is necessary to work with him as quickly as possible and always in a fume hood [1] .
Notes
- ↑ 1 2 3 4 5 6 7 8 Virgil SC, Hughes TV, Qiu D., Wang J. N- Chlorosuccinimide: [ eng ] // e-EROS Encyclopedia of Reagents for Organic Synthesis. - 2012. - DOI : 10.1002 / 047084289X.rc145.pub3 .