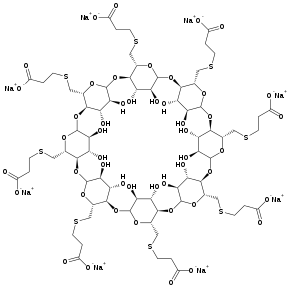
Sugammadex is a remedy for the removal of neuromuscular blockade caused by the introduction of muscle relaxants . The first "selective drug binding relaxants" (eng: SRBA ). Approved for use: European Union (2008), USA (2015) [1] .
| Sugammadex | |
|---|---|
 | |
 | |
| Chemical compound | |
| Gross formula | C 72 H 104 Na 8 O 48 S 8 |
| Molar mass | 2178 g / mol |
| CAS | |
| Pubchem | |
| Drugbank | |
| Classification | |
| ATH | |
| Mode of administration | |
| Intravenous | |
| Other names | |
| Bridion | |
Content
Pharmacological action
Modified γ- cyclodextrin .
Indications
Elimination of neuromuscular blockade caused by: rocuronium bromide , vecuronium bromide [2] .
Contraindications
Hypersensitivity .