Risperidone ( eng. Risperidone ) is an atypical antipsychotic agent, a benzisoxazole derivative developed by Janssen Pharmaceutica and first approved for use in 1993 .
| Risperidone | |
|---|---|
| Risperidonum | |
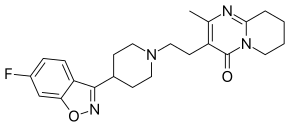 | |
| Chemical compound | |
| IUPAC | 4- [2- [4- (6-Fluorobenzo [ d ] isooxazol-3-yl) -1-piperidyl] ethyl] -3-methyl- 2,6-diazobicyclo [4.4.0] deca-1,3-dien-5-one |
| Gross formula | C 23 H 27 FN 4 O 2 |
| Molar mass | 410,485 g / mol |
| CAS | |
| Pubchem | |
| Drugbank | |
| Classification | |
| Pharmacol. Group | neuroleptics |
| ATH | |
| Pharmacokinetics | |
| Bioavailable | 70% |
| Metabolism | liver (involving CYP2D6 isoenzyme) |
| The period is half out. | 3–20 hours |
| Excretion | with urine and feces |
| Dosage Forms | |
| powder for suspension for intramuscular injection, solution for oral administration, coated tablets 1, 2, 3 and 4 mg | |
| Other names | |
| Rispolept, Leptinorm, Rezalen, Ridoneks, Risdonal, Rispen, Risperon, Rispetril, Rileptid, Rispaxol, Rispolux, Risset, Sizodon-San, Speridan, Torendo, Aridon | |
It is used most often to treat schizophrenia and other psychoses with a predominance of productive disorders ( delusions , hallucinations ) [1] , bipolar affective disorder , as well as to reduce irritability and auto-aggression in childhood autism [2] [3] .
On December 29, 2007, the term of patent protection for risperidone ended, which led to the appearance on the market of cheaper versions of the drug ( generics ).
Pharmacodynamics
It has a high affinity for serotonin 5-HT 2 and dopamine D 2 receptors . It binds to α 1 adrenoreceptors and, with a slightly lower affinity, to histamine H 1 and α 2 adrenoreceptors. Does not possess affinity to cholinergic receptors. Although risperidone is a potent D 2 antagonist (which is believed to be the main mechanism for reducing the productive symptoms of schizophrenia), it causes a slightly less pronounced inhibition of motor activity and to a lesser extent induces catalepsy than classical antipsychotics . Due to a balanced antagonism of serotonin and dopamine receptors in the central nervous system, the likelihood of extrapyramidal side effects is reduced. Risperidone may induce a dose-dependent increase in plasma prolactin concentration.
Pharmacokinetics
After ingestion, risperidone is completely absorbed from the gastrointestinal tract , C max in the blood is achieved within 1–2 hours . Food does not affect the absorption of risperidone. C SS risperidone in the body in most patients is achieved within 1 day . C SS 9-hydroxyrisperidone is achieved within 4-5 days. Plasma concentrations of risperidone are dose-proportional (in the range of therapeutic doses). Risperidone is rapidly distributed in the body, V d is 1-2 l / kg . In plasma, risperidone binds to albumin and α-1 glycoprotein. The binding of risperidone to plasma proteins is 88%, 9-hydroxyrisperidone - 77%. Risperidone is metabolized in the liver with the participation of the CYP2D6 isoenzyme of the cytochrome P450 system with the formation of 9-hydroxyrisperidone, which has a similar pharmacological action of risperidone. Antipsychotic action due to the pharmacological activity of risperidone and 9-hydroxyrisperidone. Another pathway for risperidone metabolism is N- dealkylation . T ½ risperidone from plasma is 3 hours. T ½ 9-hydroxyrisperidone and the active antipsychotic fraction is 24 hours. After 1 week of intake, 70% is excreted in the urine, 14% in the feces. In urine, the total content of risperidone and 9-hydroxyrisperidone is 35–45% . The remaining amount falls on inactive metabolites. In elderly patients and in patients with renal insufficiency, after a single oral administration, elevated plasma concentrations and delayed elimination of risperidone were observed.
Application
- Indications
- Symptomatic treatment of schizophrenia (including first-time acute psychosis, an acute attack of schizophrenia, chronic schizophrenia).
- Psychotic states with pronounced productive ( hallucinations , delusions , mental disorders, hostility, suspiciousness) and / or negative ( dull affect , emotional and social detachment, speech scarcity ) symptoms.
- To reduce affective symptoms (depression, guilt, anxiety ) in patients with schizophrenia.
- Prevention of relapses (acute psychotic states) in chronic schizophrenia.
- Treatment of behavioral disorders in patients with dementia with symptoms of aggressiveness (outbreak of anger, physical abuse), mental disorders (agitation, delusions, other psychotic symptoms).
- Treatment of mania in bipolar disorders (as a mood stabilizer as a means of adjuvant therapy).
- In 2006, the use of risperidone for symptomatic irritability therapy in children and adolescents was approved in the United States [4] .
- Contraindications
Hypersensitivity to risperidone. Contrary to the opinion that risperidone is effective in depression and obsessions , some authors argue that it is ineffective and contraindicated in these conditions [5] . Risperidone is contraindicated in Parkinson's disease , in the active period of attack in epilepsy [6] .
- Use during pregnancy and breastfeeding
Use during pregnancy is possible if the expected benefit of therapy for the mother outweighs the potential risk to the fetus. If necessary, use during lactation breastfeeding should be discontinued.
- Use in pediatrics
Data on the safety of risperidone in children under the age of 15 years are not available.
Dosing regimen
Individual. The initial dose for adults is 0.25-2 mg / day , on the 2nd day - 4 mg per day . Further, the dose can either be maintained at the same level, or, if necessary, adjusted. Typically, the optimal therapeutic dose, depending on the evidence, is in the range of 0.5-6 mg / day . In some cases, a slower dose increase and lower initial and maintenance doses may be justified.
For schizophrenia, for patients of advanced age, as well as for concomitant diseases of the liver and kidneys, an initial dose of 0.5 mg 2 times / day is recommended. If necessary, the dose can be increased to 1-2 mg 2 times / day . Maximum doses: when risperidone is used in a dose of more than 10 mg / day, there is no increase in efficacy compared with lower doses, but the risk of extrapyramidal symptoms increases. The safety of the use of risperidone in doses of more than 16 mg / day has not been studied, therefore, a further dose excess is not allowed.
In 2004, a case was described with a 38-year-old woman who survived after taking 160 mg of risperidone, 1120 mg of citalopram and 1500 mg of paracetamol for suicidal purposes [7] .
Side
Nervous system disorders: sedative effect [8] , drowsiness, fatigue, insomnia, agitation , anxiety, headache, dizziness, impaired concentration, blurred vision, extrapyramidal symptoms ( tremor , rigidity , hypersalivation , bradykinesia, akathisia , acute dystonia ) , mania or hypomania , stroke (in elderly patients with predisposing factors), hypervolemia (either due to polydipsia , or due to the syndrome of inadequate antidiuretic hormone secretion ), tardive dyskinesia (involuntary rhythmic Substantial movements of the tongue and / or face), neuroleptic malignant syndrome ( hyperthermia , muscle rigidity, autonomic instability, impaired consciousness and increased creatine phosphonase level), impaired thermoregulation, and epileptic seizures . The extrapyramidal symptoms of risperidone are much more common than other atypical antipsychotics [9] ; it is also characterized by a relatively high risk of tardive dyskinesia (in the case of the use of this drug in high doses) [10] . In patients with hyponatremia, risperidone can lead to seizures [8] . The development of depression can also be a side effect of risperidone [11] . Risperidone can cause negative disorders (in healthy volunteers, a single dose caused abulia , affective flattening and alogia ) [12] .
On the part of the digestive system : constipation, dyspepsia , nausea or vomiting, abdominal pain, increased activity of liver transaminases , dry mouth, hypo or hypersalivation , anorexia and / or increased appetite, increased or decreased body weight. It was reported abnormal liver function in young men who took risperidone, which developed following the onset of overweight; ultrasound revealed signs of fatty infiltration of the liver [13] .
On the part of the cardiovascular system: prolongation of the QT interval [14] , increase in blood pressure , with high initial doses - orthostatic hypotension and reflex tachycardia [15] .
From the side of blood-forming organs: thrombocytopenic purpura [8] , neutropenia.
On the part of the endocrine system: diabetes mellitus [16] ; often [17] [18] (according to various sources, in 35–94% of cases [18] ) - hyperprolactinemia . Hyperprolactinemia can lead to a decrease in sexual desire and impaired sexual function, amenorrhea , galactorrhea [19] , gynecomastia , decrease or absence of potency [20] , infertility [20] [21] , hypertrichosis , seborrhea of the scalp, thinning of the hair, development of osteoporosis , the occurrence of cardiovascular disorders [20] , weight gain, autoimmune disorders , water and electrolyte imbalances [17] , the risk of developing breast cancer [19] , type II diabetes [22] , pituitary tumors [23] . Mental manifestations of prolonged hyperprolactinemia may include depression, anxiety, irritability, sleep disturbances, as well as fatigue, weakness, memory loss [20] . The increase in prolactin in risperidone therapy is more pronounced than in other atypical antipsychotics (other than amisulpride ), which are most often characterized by clinically insignificant transient hyperprolactinemia [24] , and even more pronounced than in typical antipsychotics [25] . During therapy with risperidone, prolactin levels can be observed, tens of times higher than the normative values [26] . Risperidone can influence the level of prolactin even when taken in low doses [27] . During therapy with Rispoleptom Konsta (prolonged risperidone), hyperprolactinemia can persist for 6 months after discontinuation of the drug [28] .
On the part of the urogenital system: often - priapism , erectile dysfunction , ejaculatory disorders, anorgasmia, urinary incontinence, rarely - adrenal tumors.
Allergic reactions: rhinitis , skin rash [15] , angioedema , photosensitivity .
On the part of the skin: dry skin, hyperpigmentation, itching.
Others: accommodation disorders [8] , arthralgia .
Special instructions
Use with caution in patients with diseases of the cardiovascular system (including heart failure , myocardial infarction , cardiac muscle conduction disturbances, cerebrovascular accident, hypovolemia ) [15] , as well as dehydration , hypovolemia or cerebrovascular disorders . In this category of patients, the dose should be increased gradually.
The risk of developing orthostatic hypotension is especially increased in the initial period of dose selection. If hypotension occurs, a dose reduction should be considered.
Exceeding the maximum daily dose (8 mg) increases the risk of extrapyramidal disorders [15] .
When using antipsychotics, and in particular risperidone, late dyskinesia was observed. There are reports that the occurrence of extrapyramidal symptoms is one of the risk factors for the development of tardive dyskinesia. If symptoms of tardive dyskinesia appear, the abolition of all antipsychotics should be considered.
If malignant neuroleptic syndrome develops, all antipsychotics, including risperidone, should be withdrawn.
Risperidone should be used with caution in combination with other drugs of central action.
When canceling carbamazepine and other hepatic enzyme inducers, the dose of risperidone should be reviewed and, if necessary, reduced.
Metabolic and endocrine disorders
Taking risperidone, as well as some other antipsychotics, can lead to weight gain [29] and the development of diabetes mellitus [16] . For the prevention of metabolic and endocrine disorders, it is necessary to control before starting to take a neuroleptic and during it receiving body weight [30] [31] [32] and body mass index [31] [32] , fasting glucose (or hemoglobin A1c [33] ) , plasma lipid levels [19] [30] [31] [32] . Careful attention to the lifestyle and diet of the patient. Meals should be as low in calories as possible, and lifestyle should be more active; however, diet and exercise require careful dosing [34] . If substantial weight gain is noticed, the patient should be referred to a nutritionist and a physiotherapy specialist [19] . You should be wary of the possible symptoms of diabetes (weight loss, drowsiness, thirst, polyuria ). With developing diabetes, it is important to diagnose it as early as possible and begin treatment to prevent the occurrence of life-threatening conditions associated with diabetes ( acidosis and coma ) [30] .
Impact on ability to drive vehicles and control mechanisms
During the period of treatment, until the individual sensitivity to risperidone is ascertained, patients should avoid driving vehicles and other activities requiring high concentration of attention and speed of psychomotor reactions.
Drug interactions
With the simultaneous use of inducers of microsomal liver enzymes (for example, carbamazepine ), it is possible to reduce the concentration of risperidone in the blood plasma .
With simultaneous use with phenothiazine derivatives , tricyclic antidepressants and beta-adrenergic blockers , the concentration of risperidone in the blood plasma may increase [15] . Fluoxetine may also increase the concentration of risperidone in the blood plasma.
Risperidone reduces the effects of levodopa and other dopamine receptor agonists [15] . Potentiates the action of neuroleptics [6] .
When combined with benzodiazepines , an additional sedative effect may occur [35] .
Criticism
In 2012, in the United States, by a court decision, Johnson & Johnson , which produces risperdal (risperidone), was fined more than $ 1.1 billion. The jury acknowledged that the company and its subsidiary Janssen downplayed and concealed the risks associated with taking this drug, such as an increased risk of mortality, stroke , seizures , weight gain, and diabetes . A group of federal drug experts concluded that the drug was used too widely.More than a quarter of patients taking this drug, including over-the-counter, were children and adolescents. The world-famous children's psychiatrist Joseph Biederman from Harvard actively advertised the risperdal for his appointment to children and extorted money from the company [36] .
В апреле 2012 года правительство США дало ход другому делу о мошенничестве компании Johnson & Johnson: компания платила взятки организации Omnicare — самой крупной национальной аптеке для домов престарелых , стремясь принудить её закупить и рекомендовать риспердал и другие лекарства компании. При этом Johnson & Johnson скрыла от Omnicare, что FDA запретила рекламировать риспердал в качестве эффективного и безопасного препарата для пожилых пациентов, так как он не был должным образом изучен, и что она не одобрила этот препарат для лечения психотических и поведенческих проявлений деменции (самое распространённое применение в клиниках, обслуживаемых Omnicare) из-за отсутствия данных о его безопасности [36] .
Notes
- ↑ Машковский М. Д. Лекарственные средства. — 16-е изд. — М. : Новая волна, 2012. — С. 75. — 1216 с. — ISBN 978-5-7864-0218-7 .
- ↑ Eric Hollander, Evdokia Anagnostou. Clinical Manual for the Treatment of Autism . — American Psychiatric Publishing, 2007. — P. 105—106. — ISBN 978-1-58562-222-1 .
- ↑ Kirino E. Efficacy and tolerability of pharmacotherapy options for the treatment of irritability in autistic children (англ.) // Clin Med Insights Pediatr : journal. - 2014. - Vol. 8 — P. 17—30 . — DOI : 10.4137/CMPed.S8304 . — PMID 24932108 .
- ↑ FDA (October 2, 2006). FDA Approves the First Drug to Treat Irritability Associated with Autism, Risperdal. Press release. — Пресс-релиз агентства FDA , США. (eng.)
- ↑ Атипичные антипсихотики: правда и вымысел // Московская областная психиатрическая газета. — Сентябрь 2008 г. — № 5 (42) . Архивировано 24 июня 2015 года.
- ↑ 1 2 Подкорытов В. С., Чайка Ю. Ю. Депрессии. Современная терапия. — Харьков: Торнадо, 2003. — 352 с. — ISBN 966-635-495-0 .
- ↑ Türkçapar H., Tütüncü R. Suicide attempt by ingestion high doses of risperidone, citalopram and paracetamol (англ.) // Klinik Psikofarmakoloji Bulteni — Bulletin of Clinical Psychopharmacology. - 2004. - Vol. 14 , no. 1 . — P. 14—17 .
- ↑ 1 2 3 4 Клиническая психиатрия: [Учеб. пособие]: Пер. с англ., перераб. and add. / Х.И. Каплан, Б.Дж. Садок; Ред. и авт. additional Ю.А. Александровский, А.С. Аведисова, Л.М. Барденштейн и др.; Ch. ред.: Т.Б. Дмитриева. — Москва : ГЭОТАР МЕДИЦИНА, 1998. — 505 с. — ISBN 5-88816-010-5 . Оригинал: Pocket Handbook of Clinical Psychiatry / Harold I Kaplan, Benjamin J Sadock. — Baltimore: Williams & Wilkins. — ISBN 0-683-04583-0 .
- ↑ Риск развития экстрапирамидного синдрома у больных шизофренией, получающих антипсихотики: популяционное исследование // Clin. Pharmacol. Ther.. — 2007. Архивировано 2 марта 2014 года.
- ↑ Tarsy D, Lungu C, Baldessarini RJ. Epidemiology of tardive dyskinesia before and during the era of modern antipsychotic drugs // Handb Clin Neurol. — 2011. — Т. 100 . — С. 601—616 . — PMID 21496610 .
- ↑ Яворская С.А. Применение селективных ингибиторов обратного захвата серотонина в неврологической практике // Русский медицинский журнал. — 7 марта 2007 г. — № 5 . Архивировано 22 марта 2015 года.
- ↑ Park CH , Park TW , Yang JC , Lee KH , Huang GB , Tong Z. , Park MS , Chung YC No negative symptoms in healthy volunteers after single doses of amisulpride, aripiprazole, and haloperidol: a double-blind placebo-controlled trial. (англ.) // International clinical psychopharmacology. — 2012. — Vol. 27, no. 2 — P. 114—120. — DOI : 10.1097/YIC.0b013e3283502773 . — PMID 22241281 .
- ↑ Фармакотерапия в неврологии и психиатрии: [Пер. с англ.] / Под ред. С. Д. Энна и Дж. Т. Койла. — Москва: ООО: «Медицинское информационное агентство», 2007. — 800 с.: ил. with. - 4000 copies — ISBN 5-89481-501-0 .
- ↑ Бурбелло А.Т., Бабак С.В., Андреев Б.В., Колбин А.С., Горячкина К.А. Неблагоприятные побочные реакции лекарственных средств (пособие для врачей) / Под ред. А.Т.Бурбелло. — Санкт-Петербург, 2008. Архивировано 24 декабря 2014 года. Архивная копия от 4 апреля 2017 на Wayback Machine
- ↑ 1 2 3 4 5 6 Губский Ю. И., Шаповалова В. А., Кутько И. И., Шаповалов В. В. Лекарственные средства в психофармакологии. — Киев — Харьков: Здоров'я — Торсінг, 1997. — 288 с. — 20 000 экз. — ISBN 5-311-00922-5 , 966-7300-04-8.
- ↑ 1 2 Chabroux S, Haffen E, Penfornis A. Diabetes and second-generation (atypical) antipsychotics // Ann Endocrinol (Paris). — 2009 Sep. — Т. 70 , № 4 . — С. 202—210 . — DOI : 10.1016/j.ando.2009.07.003 . — PMID 19700142 .
- ↑ 1 2 Бурчинский С.Г. Проблема безопасности в стратегии фармакотерапии атипичными нейролептиками // Нейро News: психоневрология и нейропсихиатрия. — Сентябрь 2010. — № 5 (24) . Архивировано 6 октября 2014 года.
- ↑ 1 2 Горобец Л.Н., Буланов В.С., Комиссаров П.С., Ермолаева Л.Г. Проблема гиперпролактинемии при терапии антипсихотическими препаратами // Социальная и клиническая психиатрия : журнал. — 2003. — Т. 13 , вып. 1 . — С. 164—169 . — ISSN 0869-4893 .
- ↑ 1 2 3 4 Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J. Practice Guideline for the Treatment of Patients With Schizophrenia. — 2nd ed. — American Psychiatric Association, 2004. Перевод фрагмента: Применение нейролептиков при шизофрении // Стандарты мировой медицины. — 2005. — № 2/3 . — С. 83—112 . Архивировано 25 сентября 2013 года.
- ↑ 1 2 3 4 Кушнир О.Н. Гиперпролактинемия в психиатрической практике (клиническая картина, лечение, профилактика) // Психиатрия и психофармакотерапия. — 2007. — Т. 9 , № 1 . Archived February 2, 2013.
- ↑ Maguire GA. Повышение уровня пролактина при терапии антипсихотиками: механизмы действия и клинические последствия (реферат) = J Clin Psychiatry 2002; 63 (suppl. 4): 56–62 // Психиатрия и психофармакология. — 2006. — Т. 08 , № 6 . Архивировано 28 декабря 2008 года.
- ↑ Горобец Л.Н., Поляковская Т.П., Литвинов А.В. и др. Проблема остеопороза у больных с психическими расстройствами. Часть 2 // Социальная и клиническая психиатрия. — 2013. — Т. 23 , № 1 . — С. 87—92 .
- ↑ Szarfman A., Tonning JM, Levine JG, Doraiswamy PM Atypical antipsychotics and pituitary tumors: a pharmacovigilance study (англ.) // Pharmacotherapy : journal. — 2006. — June ( vol. 26 , no. 6 ). — P. 748—758 . — DOI : 10.1592/phco.26.6.748 . — PMID 16716128 . (недоступная ссылка) Перевод: Атипичные антипсихотики и опухоли гипофиза: исследование фармакобдительности
- ↑ Цыганков Б.Д., Агасарян Э.Г. Современные и классические антипсихотические препараты: сравнительный анализ эффективности и безопасности // Психиатрия и психофармакотерапия. — 2006. — Т. 8 , № 6 . Archived February 2, 2013.
- ↑ Григорьева Е.А., Рицков А.С. Особенности действия атипичного нейролептика амисульприда // Журнал неврологии и психиатрии им. С.С.Корсакова. — 2004. — № 6 . Архивировано 6 октября 2014 года.
- ↑ Горобец Л.Н., Поляковская Т.П., Литвинов А.В. и др. Проблема остеопороза у больных с психическими расстройствами. Часть 1 // Социальная и клиническая психиатрия. — 2012. — Т. 22 , № 3 . — С. 107—112 .
- ↑ Peuskens J, Pani L, Detraux J, De Hert M. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review // CNS Drugs. — 2014 May. - Vol. 28, no. 5. — P. 421-53. — DOI : 10.1007/s40263-014-0157-3 . — PMID 24677189 .
- ↑ Рациональная фармакотерапия в психиатрической практике: руководство для практикующих врачей / Под общ. ed. Ю. А. Александровского, Н. Г. Незнанова. — Москва: Литтерра, 2014. — 1080 с. — (Рациональная фармакотерапия). — ISBN 978-5-4235-0134-1 .
- ↑ Маляров С. А.; подг. М. Добрянская. Побочные реакции антипсихотических средств // Нейро News: психоневрология и нейропсихиатрия. — Январь 2010. — № 1 (20) .
- ↑ 1 2 3 Melkersson K, Dahl ML. Метаболические нарушения на фоне терапии атипичными антипсихотиками (реферат) // Психиатрия и психофармакотерапия. — 2006. — Т. 11 , № 2 . Архивировано 19 января 2013 года.
- ↑ 1 2 3 Мосолов С.Н., Рывкин П.В., Сердитов О.В., Ладыженский М.Я., Потапов А.В. Метаболические побочные эффекты современной антипсихотической фармакотерапии // Социальная и клиническая психиатрия. — Москва, 2008. — Т. 18 , вып. 3 — С. 75—90 . Архивировано 12 марта 2012 года.
- ↑ 1 2 3 Психиатрия. Национальное руководство / Под ред. Дмитриевой Т.Б., Краснова В.Н., Незнанова Н.Г., Семке В.Я., Тиганова А.С. — Москва: ГЭОТАР-Медиа, 2011.
- ↑ Маляров С. А.; подг. М. Добрянская. Побочные реакции антипсихотических средств // Нейро News: психоневрология и нейропсихиатрия. — Январь 2010. — № 1 (20) . (inaccessible link)
- ↑ Горобец Л.Н. Эндокринные побочные эффекты нейролептической терапии . — Общероссийская общественная организация инвалидов вследствие психических расстройств и их семей «Новые возможности». IV межрегиональное совещание. 17—20 апреля. Москва, 2005. Архивировано 22 февраля 2012 года.
- ↑ Взаимодействие лекарств и эффективность фармакотерапии / Л. В. Деримедведь, И. М. Перцев, Е. В. Шуванова, И. А. Зупанец, В. Н. Хоменко; by ed. проф. И. М. Перцева. — Харьков: Издательство «Мегаполис», 2001. — 784 с. - 5000 copies — ISBN 996-96421-0-Х.
- ↑ 1 2 Goetshe P. Mortally dangerous drugs and organized crime: How a big pharma has corrupted public health / [Trans. from English L. E. Ziganshina]. - Moscow: “E” Publishing House, 2016. - 464 p. - (Evidence-based medicine). - 3000 copies - ISBN 978-5-699-83580-5 .