Beta-amyloids ( eng. Amyloid beta , Aβ) is the common name for several peptides consisting of about 40 amino acid residues and formed from a transmembrane protein - the precursor of beta-amyloid . The main species are a peptide of 40 amino acid residues (Aβ40) and a peptide of 42 amino acid residues (Aβ42) [1] . Their role in normal physiology remains unknown. Aβ40 peptide does not have pathogenic properties, but Aβ42 peptide is considered one of the main factors provoking Alzheimer's disease and is often called just beta-amyloid, without specifying the length of the amino acid chain. In the brain of a patient suffering from Alzheimer's disease, this peptide may form so-called amyloid plaques, consisting of clusters of peptide rolled up in the form of beta folds . The Aβ42 peptide can also form oligomers that trigger the chain reactions of the formation of amyloid plaques and tau proteins by the prion mechanism [2] .
Structure
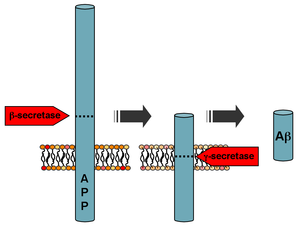
In the body, beta-amyloids are formed from the precursor of beta-amyloid ( English amyloid precursor protein , APP), a transmembrane glycoprotein with unknown functions, ranging in length from 695 to 770 amino acid residues. The proteolysis of APP with the release of beta-amyloid is performed sequentially, beta-secretase and [3] . Beta-secretase ( English β-site amyloid precursor protein cleaving enzyme 1 , BACE1) cuts the chain of amino acid residues of APP near the plasma membrane from the inside. Gamma secretase cuts the APP chain in a transmembrane region with significant variability at the site of the break, resulting in the emergence of a whole family of peptides with a chain length of 30 to 51 links [4] . The released peptides enter the blood plasma , cerebrospinal fluid or other intercellular fluids. In the cerebrospinal fluid of people not suffering from Alzheimer's disease, the ratio of major beta-amyloids is estimated at about 50% Aβ40, 16% Aβ38 and 10% Aβ42 [5] . The functions of most of these peptides remain unknown. Aβ42 peptide, which is considered one of the key pathogenic factors in the development of Alzheimer's disease, has been best studied. Its amino acid sequence is as follows [6] :
( N-End ) DAEFRHDSGYEFHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA ( C-End )
Beta amyloids are destroyed by some endopeptidases . In the brain, the most important role in maintaining the beta-amyloid balance is played by - zinc- dependent , which in a healthy body destroys the monomers and oligomers of beta-amyloids, compensating for their formation from APP. However, it is unable to destroy amyloid plaques [7] .
Role in Alzheimer's Disease
Alzheimer's disease is one of the most common neurodegenerative diseases of the elderly. Currently, there are no drugs that can slow the progression of the disease, just as there is no complete understanding of the causes of the disease. Within the framework of the most common amyloid hypothesis, it is believed that the Aβ42 peptide plays an important role in triggering irreversible changes in the patient's brain. This form is capable of forming oligomers and insoluble accumulations of a significant number of monopeptides in the beta-fold structure, which are called amyloid plaques. In the original version of the amyloid hypothesis proposed in the early 1990s by Hardy and Higgins, it was assumed that amyloid plaques cause pathological changes in the patient's brain, which are manifested in the formation of neurofibrillary tangles , impaired synaptic transmission , neuronal death and the resulting dementia [8] . According to modern concepts, Aβ42 triggers a complex set of processes at the biochemical and cellular levels, which ultimately lead to neurodegenerative changes in the brain [9] .
In 2015, studies of British physicians found that there was a danger of becoming infected with iatrogenic beta-amyloid, that is, during medical procedures such as, for example, surgery or injection . At necropsy , beta-amyloid plaques were found in the brain tissue of patients who died from Creutzfeldt-Jakob disease . The age and genetic portrait of a part of these patients ruled out spontaneous development of beta-amyloid pathologies, therefore, researchers with a high degree of probability called the cause of amyloid disease injection of growth hormone , obtained from the pituitary gland of deceased people. These injections were carried out mainly to correct growth retardation in children from 1958 to 1985, until the risk of infection with prion diseases was established.
In 2018, research results were published confirming the possibility of infection with Creutzfeldt-Jakob disease when mice were injected with beta-amyloid contaminated with human growth hormone. In the experimental group of genetically modified mice, in which the precursor of the human variant of beta-amyloid was synthesized, plaques were formed in the brain structures, while this was not observed in the control group [10] .
If the risk of infection with Alzheimer's disease during medical procedures is considered significant, this will entail serious and potentially extremely costly changes in the regulations for the decontamination of medical instruments. Beta amyloids tend to “stick” to metallic instruments, and their reliable disinfection from prions will require much more stringent conditions than from bacteria and viruses [11] .
According to animal studies, beta-amyloids can act as an antiviral [12] and antibacterial [13] brain defense mechanism. When infected with the herpes virus of mice, nerve cells begin to actively produce beta-amyloids that bind the virus, which causes the formation of amyloid plaques, but prevents the development of encephalitis [12] .
Role in sleep and memory disorders
The level of soluble beta-amyloids increases in the body during wakefulness and decreases during sleep . In studies on mice, it was found that sleep deprivation accelerates beta-amyloid accumulation in mice mutant for the beta-amyloid precursor gene (APP), and beta-amyloid accumulation in such mice disrupts sleep [14] . Disruption of the rhythm of sleep and wakefulness with age, leading to an increase in the concentration of beta-amyloid, correlates with deterioration in the quality of sleep and may be one of the mechanisms that affect memory impairment during aging and Alzheimer's disease [15] .
Medicines
To reduce the level of Aβ42, a search is under way for drugs that interfere with its formation in the brain or remove already formed plaques in the tissues. These studies are conducted in three main areas: how to prevent the formation of Aβ42, how to clear the already accumulated Aβ42 plaques, and how to prevent Aβ42 oligomerization. In 1995, researchers managed to develop a line of transgenic mice with the mutant human APP gene, in whose brain amyloid plaques accumulated [16] . These mice coped worse with tasks that required storing information, and they became a model for studying the actions of promising anti-amyloid drugs. However, so far no drugs tested in mice have shown their effectiveness in humans. One of the possible reasons for the failure of the transfer of research results in mice to humans can be the difference in the neurochemistry and pathophysiology of mouse and human neurons. In 2014, a group of scientists under the leadership of Rudolf Tanzi and Kim Du Yong managed to create a three-dimensional culture of human cells in vitro , in which the neurodegenerative changes associated with beta-amyloid and also taupathy are reproduced at an accelerated rate [17] . This achievement is considered one of the most promising in terms of rapid development and testing of drugs that can prevent the development of Alzheimer's disease in humans, and its author was included in the list of the hundred most influential people according to Time 100 in 2015 [18] .
Drug development strategies aimed at preventing the accumulation of amyloid plaques in Alzheimer's disease include reducing the concentration of amyloidogenic proteins by inhibiting or modulating secretases , especially BACE1 , proteolysis of Aβ by neplasticin or catalytic antibodies, and removing amyloids by immunization [19] .
Notes
- ↑ Gerald Karp. Cell and Molecular Biology: Concepts and Experiments. - 7th ed. - John Wiley & Sons, Inc., 2013. - P. 67. - 864 p. - ISBN 978-1118-30179-1 .
- ↑ Nussbaum Justin M., Seward Matthew E., Bloom George S. Alzheimer's disease: a tale of two prions // Prion. - 2013. - Vol. 7. - P. 14-19. - ISSN 1933-6896 . - DOI : 10.4161 / pri.22118 .
- ↑ Wilquet Valérie, Strooper Bart De. Amyloid-beta precursor protein processing in neurodegeneration // Current Opinion in Neurobiology. - 2004. - Vol. 14. - p. 582-588. - ISSN 09594388 . - DOI : 10.1016 / j.conb.2004.08.001 .
- Sson Olsson F., Schmidt S., Althoff V., Munter LM, Jin S., Rosqvist S., Lendahl U., Multhaup G., Lundkvist J. Characterization of Intermediate Steps in Amyloid Beta (Aβ) Production under Near-native Conditions // Journal of Biological Chemistry. - 2013. - Vol. 289. - P. 1540-1550. - ISSN 0021-9258 . - DOI : 10.1074 / jbc.M113.498246 .
- ↑ Bibl Mirko, Gallus Marion, Welge Volker, Lehmann Sabine, Sparbier Katrin, Esselmann Hermann, Wiltfang Jens. Characterization of cerebrospinal fluid aminoterminally truncated and oxidized amyloid-β peptides // PROTEOMICS - Clinical Applications. - 2012. - Vol. 6. - P. 163-169. - ISSN 18628346 . - DOI : 10.1002 / prca.201100082 .
- ↑ Kummer Markus P, Heneka Michael T. Truncated and modified amyloid-beta species // Alzheimer's Research & Therapy. - 2014. - Vol. 6. - P. 28. - ISSN 1758-9193 . - DOI : 10.1186 / alzrt258 .
- ↑ Huang S.-M., Mouri A., Kokubo H., Nakajima R., Suemoto T., Higuchi M., Staufenbiel M., Noda Y., Yamaguchi H., Nabeshima T., Saido TC, Iwata N. Neprilysin-sensitive Synapse-associated Amyloid-beta Peptide Oligomers Impair Neuronal Plasticity and Cognitive Function // Journal of Biological Chemistry. - 2006. - Vol. 281. - p. 17941-17951. - ISSN 0021-9258 . - DOI : 10.1074 / jbc.M601372200 .
- ↑ Hardy J., Higgins G. Alzheimer's disease: the amyloid cascade hypothesis // Science. - 1992. - Vol. 256. - p. 184-185. - ISSN 0036-8075 . - DOI : 10.1126 / science.1566067 .
- ↑ Musiek Erik S, Holtzman David M. Three dimensions of amyloid hypothesis: time, space and 'wingmen' // Nature Neuroscience. - 2015. - Vol. 18. - P. 800-806. - ISSN 1097-6256 . - DOI : 10.1038 / nn.4018 . - PMID 26007213 .
- ↑ John Collinge, Dominic M. Walsh, Sebastian Brandner, Peter Rudge, Takaomi Saido. Transmission of amyloid-β protein pathology from cadaveric pituitary growth hormone (English) // Nature. - 2018-12-13. - P. 1 . - ISSN 1476-4687 . - DOI : 10.1038 / s41586-018-0790-y .
- ↑ Abbott Alison. Autopsies reveal signs of Alzheimer's in growth-hormone patients // Nature. - 2015. - Vol. 525. - P. 165-166. - ISSN 0028-0836 . - DOI : 10.1038 / 525165a .
- ↑ 1 2 How herpes virus can cause Alzheimer's disease (rus.) . The appeal date is September 16, 2018.
- ↑ Alzheimeric protein protects against infections (rus.) . The appeal date is September 16, 2018.
- H Roh JH, Huang Y., Bero AW, Kasten T., Stewart FR, Bateman RJ, Holtzman DM Disruption of the Sleep-Wake Cycle and Diarrheal Fluoro-Amyloid in Mice with Alzheimer's Disease Pathology // Science Translational Medicine. - 2012. - Vol. 4. - P. 150ra122-150ra122. - ISSN 1946-6234 . - DOI : 10.1126 / scitranslmed.3004291 .
- ↑ Lucey Brendan P., Bateman Randall J. Alzheimer's disease pathogenesis // Neurobiology of Aging. - 2014. - Vol. 35. - P. S29-S34. - ISSN 01974580 . - DOI : 10.1016 / j.neurobiolaging.2014.03.035 .
- ↑ Games Dora, Adams David, Alessandrini Ree, et. al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein // Nature. - 1995. - Vol. 373. - p. 523-527. - ISSN 0028-0836 . - DOI : 10.1038 / 373523a0 .
- I Choi Se Hoon, Kim Young Hye, Hebisch Matthias, Sliwinski Christopher, Lee Seungkyu, d'Avanzo Carla, Chen Hechao, Hooli Basavaraj, Asselin Caroline, Muffat Julien, Klee Justin B., Zhang Can, Wainger Brian J., Peitz Michael , Kovacs Dora M., Woolf Clifford J., Wagner Steven L., Tanzi Rudolph E., Kim Doo Yeon. A three-dimensional human neural cell culture model of Alzheimer's disease // Nature. - 2014. - Vol. 515. - p. 274-278. - ISSN 0028-0836 . - DOI : 10.1038 / nature13800 .
- ↑ Maria Shriver. Alzheimer's pioneer (04/16/2015). The appeal date is July 2, 2015.
- ↑ Eisele YS, Monteiro C., Fearns C., Encalada SE, Wiseman RL, Powers ET, Kelly JW Targeting protein aggregation for degenerative diseases (Eng.) // Nat. Rev. Drug Discov .. - 2015. - Vol. 14 , no. 11 - p . 759-780 . - DOI : 10.1038 / nrd4593 .