Travoprost is a drug used in ophthalmology to reduce intraocular pressure and prevent the progression of glaucoma. Available in the form of eye drops.
| Travoprost | |
|---|---|
 | |
| Chemical compound | |
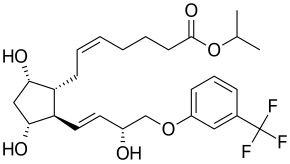
| IUPAC | propan-2-yl 7- [3,5-dihydroxy-2- [3-hydroxy-4- [3- (trifluoromethyl) phenoxy] -but-1-enyl] -cyclopentyl] hept-5-enoate |
| Gross formula | C 26 H 35 F 3 O 6 |
| Molar mass | 500.548 g / mol |
| CAS | |
| Pubchem | |
| Drugbank | |
| Classification | |
| ATH | |
| Mode of administration | |
| Eye drops | |

Travoprost is an analogue of prostaglandins that increase the reabsorption of aqueous humor , a liquid that fills the anterior and posterior chambers of the eye. The altered balance between secretion and removal of aqueous humor is the cause of ocular hypertension, which, in turn, is a major risk factor for the development of glaucoma. Ocular hypertension in the absence of treatment accelerates the development of glaucoma, which can lead to irreversible loss of vision. [1] This is a synthetic prostaglandin analog (or, more specifically, a prostaglandin F 2α analog) [2] , which works by increasing the outflow of fluid from the eyes. [3] [4] Travoprost is known for its trademarks Travatan and Travatan Z , manufactured by Alcon and Travo-Z, manufactured by Micro Labs. [four]
Side Effects
One of the strongest side effects is the darkening of the iris due to stimulation of melanocytes, which, under the action of the drug, increase the production of melanin pigment. This phenomenon is not serious for drug withdrawal, but it can potentially lead to heterochromia .
Other possible side effects are: [4]
- Possible blurred vision
- Redness of the eyelids possible
- Constant darkening of the eyelashes is possible.
- Eye irritation is possible.
- Perhaps a temporary burning sensation during use
- Eyelash thickening is possible
- Possible inflammation of the prostate gland , limiting the flow of urine ( BPH )
Other drugs
Other drugs to reduce intraocular pressure are latanoprost , brimonidine , timolol, and pilocarpine .
Notes
- ↑ Servicio Navarro de Salud: Travoprost. Ficha de Evaluación terapeutica nº 5, 2002 . Consultado el 20-5-2010
- ↑ Alcon Laboratories, Inc. TRAVATAN Z (travoprost) solution . DailyMed . Bethesda, MD: US National Library of Medicine (September 2011). The appeal date is September 30, 2011.
- ↑ AHFS Consumer Medication Information. Travoprost Ophthalmic . MedlinePlus . Bethesda, MD: US National Library of Medicine (January 1, 2011). The appeal date is September 30, 2011.
- ↑ 1 2 3 Translated from http://en.wikipedia.org/wiki/Travoprost