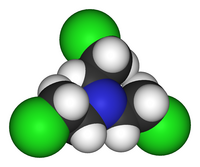
Tris (2-chloroethyl) amine is an organochlorine compound with the chemical formula N (CH 2 CH 2 Cl) 3 . Often called "HN3 gas", "HN3 substance", "nitrogen mustard HN3", "nitrogen mustard" it refers to the group of bis-B-chloroethylamine derivatives , or, in other words, to the group of nitrogen analogues of mustard gas ("mustard gas"). It was originally used as a chemical warfare agent , a component of chemical weapons .
| Tris | |
|---|---|
 | |
 | |
| Are common | |
| Chem. formula | C₆H₁₂Cl₃N |
| Physical properties | |
| Molar mass | 204.54 g / mol |
| Density | 1.24 g / ml |
| Thermal properties | |
| T. melt. | |
| T. decomp. | |
| Classification | |
| Reg. CAS number | |
| PubChem | |
| Smiles | |
| Inchi | |
| RTECS | |
| UN number | |
| ChemSpider | |
"HN3 gas" was the last in a series of nitrogen mustards developed for military purposes. It turned out to be the most successful of nitrogen mustards for this purpose, and is the only nitrogen mustard, which is still used for military purposes. “HN3 gas” is considered the main and most typical, classical representative of “military” nitrogen mustards (just like chlormethine is the most typical, classic, historically first of the antitumor derivatives of nitrogen mustards that have found application in medicine. The skin-boil effect of “HN3 gas” is approximately equivalent in strength blister the strongest of mustards , HD, and therefore the analogy between these two types of mustards as a chemical weapon components most complete [2] . Since this substance is a strong b evym toxic substance blister type of action, its production and use is strictly limited in accordance with the International Convention on the Prohibition of Chemical Weapons.
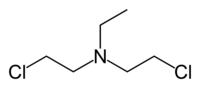 Bis- (2-chloroethyl) ethylamine , also known as “ Nitrogen mustard HN1 ”, “ HN1 gas ” or simply codenamed “ HN1 ” |
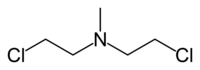 Methyl bis (2-chloroethyl) amine hydrochloride, also known as “ Nitrogen mustard HN2 ”, “ HN2 gas ” or simply codenamed “ HN2 ”, chlormethine , mechlorethamine , mustin , mustargen , embikhine |
 “Tris- (2-chloroethyl) amine hydrochloride, also known as“ Nitrogen Mustard HN3 ”,“ Gas HN3 ”,“ Substance HN3 ”or simply codenamed“ HN3 ” |
Mechanism of Action
All nitrogen mustard analogs alkylate DNA of rapidly dividing skin cells . However, for the manifestation of alkylating properties, conversion (cyclization) into the corresponding aziridinium salt is necessary . The rate of this cyclization reaction to the biologically active aziridinium salt strongly depends on the pH of the medium, since the protonated amine cannot cyclize. The aziridinium ion then reacts with water in a slower hydrolysis reaction, in which inactive compounds are formed. At pH 8 (that is, with an alkaline reaction of the medium), most of the nitrogen mustard almost immediately turns into the aziridinium salt, followed by slow hydrolysis in water. At pH 4 (that is, with an acidic reaction of the medium), on the contrary, cyclization to biologically active aziridinium occurs slowly, and subsequent hydrolysis is fast enough. And since the reaction in the body fluids and tissues is normally slightly alkaline (pH ~ 7.4-7.7), this leads to a high rate of cyclization of nitrogen mustards in the body's aqueous media to biologically active aziridinium, their rapid manifestation of an alkylating effect and the subsequent relatively slow hydrolysis.
Applications
In the early stages of the development of anticancer chemotherapy as a means of treating malignant tumors, “HN3 gas” was used for some time for this purpose, for example, for the treatment of lymphogranulomatosis and lymphoma (unlike HN1 gas, which was not particularly successful in chemotherapy of malignant tumors and from the very beginning not used for these purposes). However, he quickly gave way to a less toxic and more effective chloromethine (mechlorethamine, mustargen, “HN2 gas”). It continues to be used in dermatological practice for the treatment of psoriasis (available in the form of an ointment called “Antipsoriaticum”, diluted with ointment base in a ratio of 1: 100 000 or 1:40 000). Also, “HN3 gas” has been used for some time in experiments in the field of semiconductor chemistry. [3] However, this compound is of primary interest precisely in connection with the possibility of its use for military purposes, and it is by far the only nitrogen mustard that has retained its importance as a chemical warfare agent, a component of chemical weapons.
It should be noted that nitrogen mustards are more toxic and manifestations of their toxicity occur earlier than in "classic" sulfur mustards.
Exposure Consequences
Tris- (2-chloroethyl) amine, or “HN3 gas,” can be absorbed into the body by inhalation , by ingestion , contact with skin , mucous membranes of the eyes and other mucous membranes available, but in military use, inhalation poisoning is the most common cause of damage. Tris- (2-chloroethyl) amine is extremely toxic and, if it comes into contact with the skin, mucous membranes, respiratory system or digestive tract, causes damage to the skin and mucous membranes, cell death, blistering, ulceration, and mucous membrane or skin detachment. In addition, tris- (2-chloroethyl) amine, like other alkylating agents, very strongly inhibits bone marrow hematopoiesis and the immune system . Tris- (2-chloroethyl) amine easily and quickly penetrates the skin and mucous membranes, is rapidly absorbed by both ingestion and inhalation, and quickly binds to proteins and DNA of cells , but its effects on the human body are manifested slowly. The true degree of cell damage and the depth of immunosuppression and myelosuppression under the influence of “HN3 gas” may remain unknown for several days or even 1-2 weeks. [2]
See also
- Bis-β-chloroethylamine derivatives
- Bis- (2-chloroethyl) ethylamine , also known as “HN1 gas”, “HN1 substance”, “nitrogen mustard HN1”
- Chloromethine , or mechlorethamine, embikhin, mustine, mustargen, “HN2 gas”, “HN2 substance”, “nitrogen mustard HN2”
Links
- ↑ 1 2 http://www.cdc.gov/niosh/ershdb/emergencyresponsecard_29750012.html
- ↑ 1 2 NITROGEN MUSTARD HN-3 . Emergency Response Safety and Health Database. National Institute for Occupational Safety and Health . August 22, 2008. Accessed April 10, 2009.
- ↑ Benard, C. Chemical Vapor Deposition. - Pennington, NJ, USA: The Electrochemical Society, INC, 1997. - P. 78. - ISBN 1-56677-178-1 .