2C is the main name of the psychedelics of the phenylethylamine family, including methoxy groups at the 2nd and 5th positions of the benzene ring [1] . Most of the currently known 2C compounds were first synthesized by Alexander Shulgin in the 1970s and 1980s, and published in his book PiHKAL (“ Phenethylamines I Learned and Loved”). Dr. Shulgin also invented the term 2C, which is an acronym for 2 carbon atoms between the benzene ring and the amino group [2] .

The skeletal formula of compounds 2C

Chemical structure 2C-T-7
| Nomenclature | R3 | R4 | 2D structure | 3D structure |
|---|---|---|---|---|
| 2C-B | H | Br | 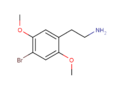 |  |
| 2C-C | H | Cl |  |  |
| 2C-D | H | CH 3 |  |  |
| 2C-E | H | CH 2 CH 3 |  |  |
| 2C-F | H | F |  |  |
| 2C-G | CH 3 | CH 3 |  |  |
| 2C-G-3 | (CH 2 ) 3 | 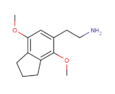 |  | |
| 2C-G-4 | (CH 2 ) 4 |  |  | |
| 2C-GN | (CH) 4 |  |  | |
| 2C-H | H | H |  |  |
| 2C-I | H | I |  |  |
| 2C-N | H | NO 2 |  | 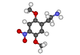 |
| 2C-O | H | Och 3 |  | 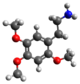 |
| 2C-O-4 | H | OCH (CH 3 ) 2 |  |  |
| 2C-P | H | CH 2 CH 2 CH 3 |  | 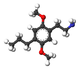 |
| 2C-SE | H | Se ch 3 |  | 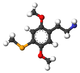 |
| 2C-T | H | SCH 3 |  | 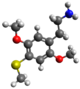 |
| 2C-T-2 | H | SCH 2 CH 3 |  |  |
| 2C-T-4 | H | SCH (CH 3 ) 2 |  |  |
| 2C-T-7 | H | S (CH 2 ) 2 CH 3 |  | 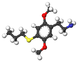 |
| 2C-T-8 | H | SCH 2 CH (CH 2 ) 2 |  |  |
| 2C-T-9 | H | SC (CH 3 ) 3 |  |  |
| 2C-T-13 | H | S (CH 2 ) 2 OCH 3 |  | 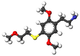 |
| 2C-T-15 | H | SCH (CH 2 ) 2 |  |  |
| 2C-T-17 | H | SCH (CH 3 ) CH 2 CH 3 |  |  |
| 2C-T-21 | H | S (CH 2 ) 2 F |  |  |
| 2C-TFM | H | CF 3 |  |  |
See also
- 25-NB
Notes
- ↑ Victor R. Preedy. Neuropathology of Drug Addictions and Substance Misuse Volume 3: General Processes and Mechanisms, Prescription Medications, Caffeine and Areca, Polydrug Misuse, Emerging Addictions and Non-Drug Addictions . - Elsevier Science, 2016 .-- P. 936. - ISBN 978-0-12-800677-1 .
- ↑ Alexander Shulgin, Ann Shulgin. PiHKAL: A Chemical Love Story. - Berkeley, California: Transform Press, 1991 .-- ISBN 0-9630096-0-5 .
Links
- Part 2 PiHKAL (English) .