The Koenig reaction is the interaction of pyridine or its derivatives not substituted at the α-positions with chlorine and bromine cyanide, leading to the formation of N-cyanopyridinium salts. Subsequent hydrolysis of these salts proceeds with the opening of the pyridine ring, and glutaconic aldehyde is formed (hydrolysis of unsubstituted N-cyanopyridinium)
In the case of substituted pyridines, substituted derivatives of glutaconic aldehyde are formed, which are usually not isolated, but are introduced in situ with nucleophiles - amines or compounds with an activated methylene group:
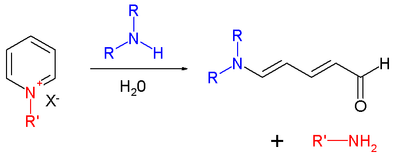
- R '= CN
It was discovered by V. Koenig, who described in 1904 the formation of a new red-violet dye during the interaction of N-cyanopyridinium bromide with anthranilic acid under conditions of alkaline hydrolysis [1] .
Application
The interaction of the aldehydes formed in the Koenig reaction with amines or compounds with an activated methylene group (e.g., barbituric acid ) leads to the formation of brightly colored compounds, which is used in the photocolorimetric determination of natural cyanides and α-unsubstituted pyridines (e.g., nicotine and anabazine ).
Various components are used as components reacting with glutaconic aldehyde (or its derivatives) to form a dye.
So, in the determination of cyanides, pyridine is used in combination with oxoheterocycles condensing with glutaconic aldehyde - barbituric acid [2] , 1-phenyl-3-methyl-5-pyrazolone [3] . When determining cyanides in tissues, blood, or other samples, the microdiffusion method is usually used, in which the sample is treated with chloramine T , which reacts with cyanides to form volatile chlorocyanin , which is absorbed in the microdiffusion cell by pyridine.
When determining nicotine, aniline is used [4] , anabazine is barbituric acid [5] , in this case alkaloids are usually isolated from the sample by steam distillation or extraction .
The disclosure of the pyridine cycle according to Koenig is also used in synthetic organic chemistry, for example, in the synthesis of indoles from 2- (2-aminophenyl) pyridines [6] :
See also
- Zink reaction
Notes
- ↑ König, W. Über eine neue, vom Pyridin derivierende Klasse von Farbstoffen (German) // Journal für Praktische Chemie : magazin. - 1904. - 28 Januars ( Bd. 69 , Nr. 1 ). - S. 105-137 . - ISSN 1521-3897 . - DOI : 10.1002 / prac.19040690107 .
- ↑ Kramarenko V.F. Chapter III, § 3 . // Toxicological chemistry. - K .: The head publishing house of the Vysha Shkola Publishing Association, 1989. - P. 188.
- ↑ Suzuki, Osamu. Drugs and poisons in humans: a handbook of practical analysis. - Birkhäuser, 2005 .-- P. 113-117. - ISBN 9783540222774 .
- ↑ International Encyclopedia of Pharmacology and Therapeutics. Section 114: Nicotine and the Tobacco Smoking Habit, page 2 . Date of treatment January 24, 2012. Archived on October 5, 2012.
- ↑ Kramarenko V.F.Chapter V, § 38 . // Toxicological chemistry - K .: Head Publishing House of the Publishing Association "Vyscha Shkola", 1989. - P. 188.
- ↑ Kearney, Aaron M; Christopher D Vanderwal. Synthesis of Nitrogen Heterocycles by the Ring Opening of Pyridinium Salts (Eng.) // Angewandte Chemie International Edition : journal. - 2006 .-- 27 November ( vol. 45 , no. 46 ). - P. 7803-7806 . - ISSN 1521-3773 . - DOI : 10.1002 / anie.200602996 .

