Benfotiamine ( novolate. Benfotiamine ) (S-benzoylthiamine-O-monophosphate) is a fat-soluble analogue of vitamin B1 ( thiamine ).
| Benfotiamine | |
|---|---|
| Benfotiamine | |
 | |
 | |
| Chemical compound | |
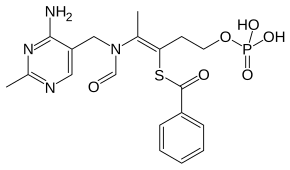
| IUPAC | S - [(2 Z ) -2 - {[(4-amino-2-methylpyrimidin-5-yl) methyl] (formyl) amino} -5- (phosphonooxy) pent-2-en-3-yl] benzenecarbothioate |
| Gross formula | C 19 H 23 N 4 O 6 PS |
| Molar mass | 466.448 g / mol |
| Cas | |
| PubChem | |
| Classification | |
| Farmakol. Group | Vitamins : Vitamins of group B |
| ATX | |
| Pharmacokinetics | |
| Bioavailable | ? |
| Plasma Protein Binding | 80% of thiamine is in red blood cells (mainly in the form of TDF), free plasma thiamine binds predominantly to albumin . The level of thiamine in whole blood ranges from 5-12 mcg / 100 ml. |
| Metabolism | 50% unchanged or thiamine sulfate ester, thiamic acid, methylthiazole acetic acid, pyramine, benzoic acid, hippuric acid |
| The half-life. | a phase - 5 hours |
| Excretion | Kidney |
| Dosage Forms | |
| Pills , Dragees | |
| Route of administration | |
| orally | |
| Other names | |
| Combilipen Tabs, Milgamma, Benfogamma, Unigamma | |
Content
- 1 Physical properties
- 2 Pharmacological action
- 3 Pharmacokinetics
- 3.1 Metabolism and distribution
- 4 Application
- 4.1 Indications
- 4.2 Contraindications
- 4.3 Special instructions
- 5 Method of application
- 6 Side effects
- 7 Holidays from pharmacies
- 8 References
Physical Properties
Benfotiamine is a colorless and odorless substance. Fat soluble; sparingly soluble in water, ethyl alcohol , chloroform , methanol and dioxane , but it is better soluble in a solution of hydrochloric acid and glacial acetic acid. The isoelectric point is in the pH range of 4.06. Benfotiamine is stable in an acidic environment and in aqueous solution. It is not hygroscopic and immune to the action of thiaminase l and II. For benfotiamine, most of the incompatibilities inherent to thiamine are uncharacteristic. Only in combination with aminophylline , vitamin C , vitamin B2 , at high temperatures, as well as at high humidity, the color of the substance underwent changes.
Pharmacological action
A synthetic compound similar in structure and action to thiamine and cocarboxylase ; It has a B1-vitamin and metabolic effect. It normalizes carbohydrate metabolism , helps normalize the function of the nervous system, and makes up for vitamin B1 deficiency. Vitamin B1 is involved in metabolism, neuro-reflex regulation, has an effect on the conduct of nervous excitation in cholinergic synapses. The active form of vitamin B1 is its derivative - cocarboxylase , which performs an important function in the carbohydrate and energy metabolism of nervous and muscle tissues.
Pharmacokinetics
After ingestion, benfotiamine in an unchanged form reaches the upper parts of the small intestine, where its dose-proportional absorption occurs. In this case, the monophosphate group is cleaved under the action of phosphatase of the intestinal mucosa, and the lipophilic properties of the molecule (due to the benzene ring) begin to prevail. Thus formed S-benzoylthiamine (S-BT) can freely passively diffuse through the cells of the mucous membrane and enter unchanged into the blood.
Metabolism and distribution
Benfotiamine is a prodrug with properties similar to thiamine, which, after absorption inside the cells, turns into the biologically active coenzyme form thiamine diphosphate (TDF). The total thiamine content in the human body is approximately 30 mg. The highest amounts of thiamine are found primarily in the brain, liver, heart, kidneys, and skeletal muscle. Thiamine in the human body is contained mainly in the form of its phosphorus esters. The predominant part is TDF, the smaller part is TMP (thiamine monophosphate) and TTF (thiamine triphosphate). The cerebrospinal fluid contains only free thiamine and TMF. This suggests that, along with free thiamine, TMF can also penetrate through cell membranes.
In whole blood, about 80% of thiamine is in red blood cells (mainly in the form of TDF), free plasma thiamine binds predominantly to albumin. The level of thiamine in whole blood ranges from 5 to 12 mcg / 100 ml.
In one of the experimental studies, after intraperitoneal administration of labeled benfotiamine or thiamine hydrochloride, the method of autoradiography determined its distribution to various organs and systems of laboratory mice ( liver , blood , brain , muscles , kidneys ). After the use of benfotiamine in all organs, a significantly higher level of radioactivity was detected than after administration of thiamine hydrochloride. These differences were most related to muscles and the brain. After using thiamine hydrochloride in the muscle tissue and brain, only about 0.2% of the level in the liver was noted, while after taking benfotiamine - about 20% of this level. These results indicate that the use of benfotiamine can achieve a significantly higher level of active substance in tissues than the use of water-soluble thiamine.
The elimination of thiamine from the body is approximately 50% unchanged or in the form of sulfate ester; the remainder are, along with as yet unidentified metabolites, mainly thiamic acid , methylthiazole acetic acid and pyramine . In the process of enzymatic conversion of benfotiamine to thiamine, benzoic and hippuric acids are additionally formed, which are excreted in the urine. Excretion occurs in 2 phases: the initial fast (a-phase) and the second, slower phase (p-phase). The half-life of benfotiamine in the a phase is 5 hours, in the p phase it is 16 hours.
Application
Indications
Hypo- and vitamin deficiency B1 (“ beriberi ”), atherosclerosis , coronary heart disease , myocardial dystrophy , disturbance of coronary circulation, rheumatic heart disease , heart failure , viral hepatitis , poisoning, disorders of the nervous system, polyneuropathy , neuritis , sciatica , neuralgia , peripheral paresis ; intestinal atony , thyrotoxicosis , endarteritis , pruritus of various etiologies, pyoderma , lichen , psoriasis , eczema , chronic gastritis , intoxication.
Contraindications
Hypersensitivity to benfotiamine.
Special instructions
Allergic reactions more often develop in women in the menopausal and postmenopausal periods, as well as in people suffering from chronic alcoholism .
Benfotiamine has an extremely low toxicity, even less than that of the water-soluble thiamine hydrochloride.
Method of use
Inside , after eating, 1-4 times a day. Adults - 25-50 mg 1-4 times a day after meals (100-200 mg / day). The course of treatment is 15-30 days. Children from 1 year to 10 years - 10-30 mg / day for 10-20 days; children over 10 years old - 30–35 mg / day for 15–30 days; persons of advanced and senile age - 25 mg 1-2 times a day.
The daily requirement for vitamin B1 for adult men is 1.2–2.1 mg; for the elderly - 1.2-1.4 mg; for women - 1.1-1.5 mg; for children - 0.3-1.5 mg.
Side
Allergic reactions: angioedema , urticaria , pruritus.
Holidays from pharmacies
OTC drug (there is even an inscription on the cardboard packaging about this).
Links
- Thiamine
- Sulbutiamine
- Take it
- Vitamins
- http://medi.ru/doc/1705071.htm