Sodium sulfate , sodium sulfate, Na 2 SO 4 - sodium salt of sulfuric acid .
| Sodium sulfate | |
|---|---|
 | |
 | |
| Are common | |
| Systematic name | sodium sulfate, sodium sulfate |
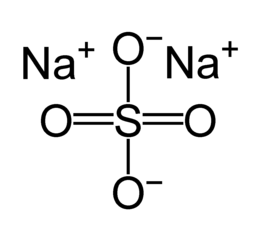
| Chem. formula | Na 2 SO 4 |
| Physical properties | |
| condition | white hygroscopic crystals |
| Molar mass | 142.04 g / mol |
| Density | 2.68 g / cm³ |
| Thermal properties | |
| T. melt. | 883 ° C |
| Education enthalpy | −1387.9 kJ / mol |
| Chemical properties | |
| Water solubility | 19.2 (20 ° C), 42.3 (100 ° C) |
| Classification | |
| Reg. CAS number | 7757-82-6 |
| Pubchem | |
| Reg. EINECS number | |
| SMILES | |
| Inchi | |
| Codex Alimentarius | |
| RTECS | |
| CHEBI | |
| Chemspider | |
Content
Properties
Colorless crystals. Anhydrous Na 2 SO 4 is stable above a temperature of 32.384 ° C, below this temperature, in the presence of water, crystalline Na 2 SO 4 · 10H 2 O is formed.
Being in nature
In nature, anhydrous sodium sulfate is found in the form of the mineral tenardite . Crystal hydrate Na 2 SO 4 · 10H 2 O forms the mineral mirabilite ( Glauber's salt ). There are also double salts of sodium sulfate with other sulfates, for example, astrakhanite Na 2 SO 4 · MgSO 4 · 4H 2 O, glauberite Na 2 SO 4 · CaSO 4 . Significant amounts of sodium sulfate are contained in brine and bottom sediments of salt lakes of chloride-sulphate type and Kara-Bogaz-Gol gulf . In them, when the temperature drops, the reaction goes:
- 2NaCl + MgSO 4 ⇆ MgCl 2 + Na 2 SO 4
In Russia, the largest producer of natural sodium sulphate is Kuchuksulfate, OJSC - 600 thousand tons per year.
Getting
The industrial method of producing sodium sulfate is the interaction of NaCl with H 2 SO 4 in special “sulphate” furnaces at 500–550 ° C; hydrogen chloride at the same time.
Currently, this method is practically not used, since there are fairly large reserves of natural raw materials.
Sodium sulphate is also obtained as waste (odorless) in the production of chromic.
Application
In the world, a large amount of sodium sulphate was used earlier in the production of synthetic detergents , but in many countries in recent years there has been a shift to concentrated (compact) laundry detergents , in which sulphate is either not used or used in small quantities. In Russia, manufacturers of laundry detergents buy more than 300 thousand tons of sodium sulfate.
The second largest use of sodium sulfate is glass production. Also, this substance is used in large quantities in the preparation of cellulose by the sulphate method, as well as in the textile , leather industry and non-ferrous metallurgy .
Sodium sulfate is used in small amounts in chemical laboratories as a dehydrating agent. Despite the fact that it dehydrates organic solvents more slowly than magnesium sulphate, many people prefer this remedy for two reasons: cheap and easy to filter.
In even smaller quantities previously used in medicine and veterinary medicine as a saline laxative agent and as a component in the means for washing the nose.
Registered as a food additive E514 .
Acidity regulator, used as a buffer additive to maintain the pH at a certain level [1] .
See also
- Glauber's salt
Notes
Literature
- Remy G. Course of inorganic chemistry. T.2. - M., 1966