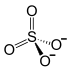
Aluminum sulfate is a complex inorganic compound , aluminum salt and sulfuric acid with the chemical formula Al 2 ( S O 4 ) 3 . It looks like colorless crystals, it can form crystalline hydrates with different water content. It is used in water purification, dyeing fabrics, tanning leather, as a reagent in photography, to produce alum .
| Aluminum sulphate | |
|---|---|
 | |
| Are common | |
| Traditional names | aluminum sulphate |
| Chem. formula | Al 2 (SO 4 ) 3 |
| Physical properties | |
| condition | solid |
| Molar mass | 342.15 g / mol |
| Density | 2.710 g / cm 3 (anh.) 1,690 g / cm 3 ( 18-aq. ) |
| Thermal properties | |
| T. melt. | |
| T. Kip. | |
| T. | 580 ° C |
| Classification | |
| Reg. CAS number | 10043-01-3 |
| Pubchem | |
| Reg. EINECS number | |
| SMILES | |
| Inchi | |
| Reg. EC number | 233-135-0 |
| Codex Alimentarius | |
| RTECS | |
| CHEBI | |
| Chemspider | |
| Security | |
| Toxicity | low toxicity |
Physical Properties
Colorless crystals, plates or powder. It has an orthorhombic lattice. Density - 2.710 g / cm 3 , specific heat capacity at constant pressure - 259.6 J / (mol K). Poor soluble in alcohols, soluble in water, hygroscopic. Stable at ordinary temperature [3] [4] .
Forms crystalline hydrate with the formula Al 2 (SO 4 ) 3 · 18H 2 O, which looks like colorless crystals, melting at 86.5 ° C (with decomposition) and with a density of 1.690 g / cm 3 . During long-term storage, 18-water crystalline hydrate can partially erode to a content of 14-14.5 water molecules. 18-water crystalline hydrate loses water when heated, forming the form [3] :
- 150 ° C - Al 2 (SO 4 ) 3 · 14 H 2 O,
- 160 ° C - Al 2 (SO 4 ) 3 · 10 H 2 O,
- 250 ° C - Al 2 (SO 4 ) 3 · 3 H 2 O,
- 420 ° C - completely anhydrous.
Chemical Properties
Aluminum sulfate decomposes at temperatures above 580 ° C into a γ-modification of alumina and sulfuric anhydride [3] :
Gelatinous photofilms are hardened, which is used in the production of photographic materials to increase the mechanical strength of the emulsion layers, and in color photography also to protect dyes from hydrolytic decomposition. The mechanism of tanning is due to the binding of aluminum ions ionized carboxyl groups of gelatin [4] [5] .
Getting
Aluminum sulfate is obtained by the interaction of aluminum hydroxide with sulfuric acid :
Also aluminum sulfate is obtained by the interaction of aluminum with sulfuric acid:
Application
Aluminum sulphate is used as a coagulant for household and industrial water purification and for use in paper, textile, leather and other industries.
Used as a food additive E-520 [6] .
In the photo is included in the compositions of stabilizing solutions and tanning fixatives [4] .
Notes
- ↑ Aluminum sulfate
- ↑ Aluminum sulfate
- ↑ 1 2 3 Volokhov, 1988 .
- ↑ 1 2 3 Gurlev, 1988 , p. 285.
- ↑ James, 1980 , p. 83
- ↑ E-520 (E-520) Aluminum Sulphate
Literature
- Volokhov, Yu.A. Aluminum sulphate: article // Chemical Encyclopedia / Redcol .: Knunyants I.L. et al. - M .: Soviet Encyclopedia , 1988. - V. 1: A — Darzan. - p. 121. - 623 p.
- Gurlev D.S. Reference photographs (photo processing). - K .: Tehnika, 1988. - 335 p. - ISBN 5-335-00125-4 .
- James T. The Theory of the Photographic Process = The theory of the photographic process. 4th American ed. by ed. Kartuzhansky A.L. - 2nd Russian ed. - L .: "Chemistry". Leningrad branch., 1980. - 672 p.

![{\ displaystyle \ mathrm {\ \ \! \ {\ Biggr]} _ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/574adab1409cb81da6c38bb738ad111e61bbb2d9)
![{\ displaystyle \ mathrm {\ \ \! \ {\ Biggr]} _ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cd32744e9f4314d390f5ee107a8bd812d0cc2da0)