Calcium sulfide is an inorganic binary chemical compound with the formula Ca S.
| Calcium sulphide | |
|---|---|
 | |
| General | |
| Systematic name | calcium sulfide |
| Traditional names | calcium sulfide, calcium sulfide |
| Chem. formula | CaS |
| Physical properties | |
| condition | white hygroscopic powder |
| Molar mass | 72.143 g / mol |
| Density | 2.59 g / cm³ |
| Thermal properties | |
| T. melt. | 2525 ° C |
| Like heat resistant. | 47.51 J / (mol · K) |
| Enthalpy of Education | −476.98 kJ / mol |
| Chemical properties | |
| Solubility in water | sparingly soluble |
| Solubility in other substances | insoluble in alcohol , reacts with acids |
| Optical properties | |
| Refractive index | 2.137 |
| Structure | |
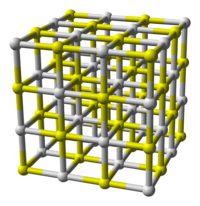
| Coordination geometry | octahedral (Ca 2+ ); octahedral (S 2− ) |
| Crystal structure | cubic ( halite ) |
| Classification | |
| Reg. CAS number | 20548-54-3 |
| PubChem | |
| Reg. EINECS number | |
| Smiles | |
| Inchi | |
| Reg. EC number | 243-873-5 |
| Chebi | |
| ChemSpider | |
| Security | |
| Toxicity |  one 2 one   |
Content
- 1 Receiving
- 2 Physical properties
- 3 Chemical properties
- 4 Application
Getting
The well-known mineral is oldhamite ( English Oldhamite ) consisting of calcium sulfide with impurities of magnesium , sodium , iron , copper . The crystals are pale brown, turning into dark brown.
Direct synthesis of elements:
The reaction of calcium hydride in hydrogen sulfide :
From calcium carbonate :
Reduction of calcium sulfate :
Physical Properties
White crystals, cubic face-centered lattice of NaCl type (a = 0.6008 nm). It decomposes upon melting. In the crystal, each S 2– ion is surrounded by an octahedron consisting of six Ca 2+ ions , while each Ca 2+ ion is surrounded by six S 2– ions.
Slightly soluble in cold water, does not form crystalline hydrates. Like many other sulfides, calcium sulfide in the presence of water undergoes hydrolysis and has the smell of hydrogen sulfide .
Chemical Properties
When heated, decomposes into components:
In boiling water it is completely hydrolyzed:
Diluted acids displace hydrogen sulfide from salt:
Concentrated oxidizing acids oxidize hydrogen sulfide:
Hydrogen sulfide is a weak acid and can be displaced from salts even by carbon dioxide:
With an excess of hydrogen sulfide, hydrosulfides are formed:
Like all sulfides, calcium sulfide is oxidized by oxygen:
Application
Used for the preparation of phosphors , as well as in the leather industry for removing hair from skins, it is also used in the medical industry as a homeopathic remedy .