Boric acid (orthoboric acid or lat. Acidum Boricum ) is a weak , monobasic Lewis acid , often used as an insecticide , antiseptic , flame retardant , neutron absorber or precursor to produce other chemical compounds. It has the chemical formula H 3 BO 3 (or B (OH) 3 ).
| Boric acid | |
|---|---|
 | |
 | |
 | |
| Are common | |
| Systematic name | Orthoboric acid |
| Chem. formula | H 3 BO 3 |
| Physical properties | |
| condition | solid |
| Molar mass | 61.83 g / mol |
| Density | 1,435 (15 ° C) |
| Thermal properties | |
| T. melt. | 170.9 ° C, 444 K, 340 ° F |
| T. bale. | 300 ° C, 573 K, 572 ° F ° C |
| Chemical properties | |
| pK a | 9.24 (I), 12.74 (II), 13.80 (III) |
| Solubility in water | 2.52 (0 ° C) g / 100 ml |
| Classification | |
| Reg. CAS number | 10043-35-3 |
| PubChem | |
| Reg. EINECS number | |
| Smiles | |
| Inchi | |
| Codex Alimentarius | |
| RTECS | |
| Chebi | |
| ChemSpider | |
| Security | |
| Toxicity |  0 2 0 |
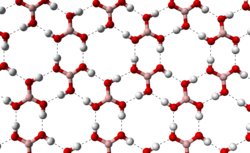
A colorless crystalline substance in the form of flavourless flakes, has a layered triclinic lattice in which the acid molecules are connected by hydrogen bonds in flat layers, the layers are interconnected by intermolecular bonds, the length of which is 272 pm. The distance between adjacent layers is 318 pm.
Metaboric acid (HBO 2 ) is also colorless crystals. It exists in three versions - the most stable γ-HBO 2 with a cubic lattice, β-HBO 2 with a monoclinic lattice and α-HBO 2 with a rhombic lattice.
When heated, orthoboric acid loses water and first passes into metaboric acid, then into tetraboric H 2 B 4 O 7 . With further heating, it is dehydrated to boric anhydride .
Aqueous solutions of boric acid are a mixture of polyboric acids of the general formula H 3m-2n B m O 3m-n . It is found in nature in the form of the sassolin mineral.
Content
Being in nature
In nature, free boric acid is found in the form of the sassolin mineral , in hot springs and mineral waters .
Getting
Boric acid can be obtained by mixing borax ( sodium tetraborate ) with mineral acid, for example, hydrochloric :
It is also a product of the hydrolysis of diborane or boron trihalides [1] :
Properties
Boric acid exhibits very weak acidic properties. It is relatively slightly soluble in water. Its acidic properties are caused not by the removal of the H + proton, but by the addition of a hydroxyl anion:
-
- K a = 5.8 × 10 −10 mol / L; p K a = 9.24.
-
It is easily displaced from solutions of its salts by most other acids. Its salts, called borates, are usually produced from various polyboric acids, most often tetraboric Н 2 В 4 О 7 , which is a much stronger acid than orthoboric.
Very weak signs of amphoteric B (OH) 3 manifests itself, forming an unstable boron hydrosulfate B (HSO 4 ) 3 .
When neutralizing orthoboric acid with alkalis , orthoborates containing the (BO 3 ) 3– ion are not formed in aqueous solutions, since orthoborates are almost completely hydrolyzed due to the too low formation constant [B (OH) 4 ] - . In the solution tetraborates, metabolites or salts of other polyboric acids are formed:
- Excess alkali, they can be converted into metabolites:
Meta- and tetraborates are hydrolyzed , but to a lesser extent (reactions opposite to those given).
The following equilibria are established in acidified aqueous solutions of borates:
When heated, boric acid dissolves metal oxides, forming salts.
With alcohols in the presence of concentrated sulfuric acid forms esters :
The formation of boromethyl ether B (OCH 3 ) 3 is a qualitative reaction to H 3 BO 3 and salts of boric acids; when ignited, the boromethyl ether burns with a beautiful bright green flame.
Boric acid in medicine
Boric alcohol ( lat. Solutio Acidi borici spirituosa ) - a solution of boric acid in ethanol (usually in 70% ethanol).
Alcoholic solutions of boric acid in a concentration of 0.5%, 1%, 2%, 3%, 5% are prepared with 70% ethanol and are used as an antiseptic and as an antipruritic agent when rubbing healthy skin around the foci of pyoderma , as well as ear drops.
Boric acid can only be dangerous if it is taken uncontrollably. A dangerous concentration in the human body (and especially the child) can occur with regular use. The lethal dose for poisoning through the mouth for an adult is 15-20 g, for children - 4-5 g [2] .
Boric acid has been used in medicine since the 1860s as an antiseptic that does not irritate wounds and has no taste, smell or color. In modern medicine, the antimicrobial effectiveness of boric acid is considered low.
The use of boric acid as an antiseptic for children, as well as pregnant and lactating women was prohibited on February 2, 1987 by the USSR Ministry of Health on the recommendation of the Pharmacological Committee with the wording: “... prohibit the use of boric acid as an antiseptic in infants, as well as women during pregnancy and lactation due to its low activity and high toxicity ” [3] .
Application
- In nuclear reactors as a neutron absorber dissolved in a coolant .
- Boric fertilizer .
- In laboratories, they are used to prepare buffer solutions .
- In medicine - as an independent disinfectant for adults , as well as in the form of a 2% solution - for washing the skin after contact with alkalis.
- Also, various combined preparations are produced on the basis of boric acid (ATX group ), for example , Teymurov paste .
- In the photograph - as a part of fine-grained developers and acid fixers to create a weak acidic environment.
- In the food industry, it is registered as food additive E284 (in Russia this additive is not included in the list of approved for use [4] ).
- In jewelry - as the basis of fluxes for soldering gold-containing alloys.
- In foundry, it is a binder for acid lining of furnaces, a component for protecting the jet from oxidation during casting of magnesium alloys.
- In everyday life - the destruction of cockroaches , ants, bugs.
- In the production of ceramics , optical fiber , fiberglass , glass [5] ,
- As a flame retardant to protect wood,
- As part of electrolytes for copper plating and nickel plating .
Notes
- ↑ Housecroft, CE Chapter 13: The Group 13 Elements // Inorganic Chemistry / CE Housecroft, AG Sharpe. - 3rd. - Pearson, 2008. - P. 340. - ISBN 978-0-13-175553-6 .
- ↑ Harmful substances in industry. Handbook for chemists, engineers and doctors / ed. prof. N.F. Lazarev. - L: Chemistry, 1977. - T. 3. - S. 310. - 608 p.
- ↑ Prozorovsky V. Insidious boric acid (Russian) // Science and life. - 2003. - No. 11 .
- ↑ SanPiN 2.3.2.1293-03.
- ↑ APPLICATION OF BORIC ACID.
Links
- Prozorovsky V. Insidious boric acid (Russian) // Science and life. - 2003. - No. 11 .
Literature
- Karapetyants M. Kh. Drakin S. I. General and inorganic chemistry. M .: Chemistry 1994
- Remy G. “The course of inorganic chemistry” M.: Foreign literature, 1963
- M. D. Mashkovsky. Medicines - M .: New Wave LLC, 2002. - T. 2. - 608 p. - 25,000 copies. - ISBN 5-7864-0129-4 .