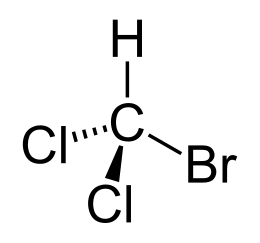
Bromodichloromethane is an organic compound with the formula CHCl 2 Br.
| Bromodichloromethane | |
|---|---|
 | |
| Are common | |
| Systematic name | bromodichloromethane |
| Chem. formula | CHBrCl 2 |
| Rat formula | CHBrCl 2 |
| Physical properties | |
| Molar mass | 163.8 g / mol |
| Density | 1.980 g / cm³ |
| Thermal properties | |
| T. melt. | -57 ° C |
| T. bale. | 90 ° C |
| Steam pressure | |
| Chemical properties | |
| Solubility in water | at 20 ° C 4.5 g / 100 ml |
| Classification | |
| Reg. CAS number | 75-27-4 |
| PubChem | |
| Reg. EINECS number | 200-856-7 |
| Smiles | |
| Inchi | |
| RTECS | |
| Chebi | |
| ChemSpider | |
| Security | |
| LD 50 | oral mouse [rat]: 450mg / kg [430mg / kg] |
| Toxicity | high, carcinogen, mutagen, teratogen, etc. |
It is contained as an admixture in methanol , in very small but sufficient quantities - in cigarettes and in tap water .
Toxic . It has a carcinogenic effect. Refers to substances of hazard class 1 .
Application
As a solvent for fats. Due to its high density, it was used for the separation of minerals. Previously, it was not he who was used for fire extinguishing, but CH2BrCl bromochloromethane (CAS: 74-97-5; https://chem.nlm.nih.gov/chemidplus/rn/74-97-5 ).
Laboratory method for producing bromodichloromethane (CHBrCl2): bromination of chloroform, in the presence of aluminum, significantly less (LD50: mouse oral 4300mg / kg) hazardous bromochloromethane CH2BrCl, anesthetic and fire fighting agent come out of dichloromethane.