T-tubules ( English T-tubules from the English transverse tubules - transverse tubules) - invaginations of the cell membrane , reaching the central part of the cells of skeletal and cardiac muscles . The T-tube membrane contains a large number of ion channels , , and pumps, so they provide fast transmission of the action potential and play an important role in regulating the intracellular concentration of calcium ions . By providing simultaneous release of calcium from intracellular depots, T-tubes provide a stronger reduction of myocytes. In some diseases, the function of the T-tubules is impaired, which in the case of cardiac muscles can lead to arrhythmias and heart attack . T-tubes were first described in 1897.
Content
Structure
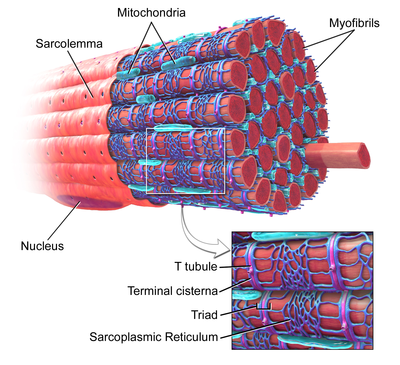
T-tubules are bulges of the plasma membrane of a muscle cell ( sarcolemma ). In each muscle cell, they form a network of tubules located perpendicular or parallel to the sarcolemma. The inner part of the T-tubes opens with an opening on the surface of the cell, which is why the T-tubes are filled with the same fluid that surrounds the cell. The T-tube membrane contains many , sodium-calcium exchangers , and β-adrenergic receptors [1] .
In atrial and ventricular cardiomyocytes, T-tubules appear during the first few weeks of life [2] . In most species, they are found in the muscle cells of the ventricles , and in large mammals - in the muscle cells of the atria [3] . The diameter of T-tubules in cardiomyocytes is from 20 to 450 nm ; as a rule, T-tubules are located in the region of Z-disks , where cellular actin filaments anchor [1] . In cardiomyocytes, T-tubules are closely related to the intracellular calcium depot — the sarcoplasmic reticulum , namely, its terminal cisterns. The complex of the T-tube and the terminal tank is called the [4] .
In skeletal muscle, T-tubes have a diameter of 20 to 40 nm and are usually located on both sides of the myosin band, at the junction of the A and I bands. In the muscles of the T-tube are connected with two terminal tanks of the sarcoplasmic reticulum, this complex is called the [1] [5] .
The shape of T-tubules is supported by a variety of proteins . The amphiphysin-2 protein encoded by the gene is responsible for the formation of T-tubules and the localization of necessary proteins, such as L-type calcium channels [6] . Junktophilin-2, encoded by the gene, is involved in the formation of the T-tube connection with the sarcoplasmic reticulum, which is necessary for the simultaneous reduction of cell sarcomeres. , encoded by the TCAP gene, is involved in the formation of T-tubes and is possibly responsible for the increase in the number of T-tubes in growing muscle [4] .
Functions
Electromechanical pairing
T-tubes are an important link on the path from the electrical excitation of a muscle cell to its muscle contraction (electromechanical conjugation). When a muscle needs to contract, a stimulating electrical signal coming from a nerve or an adjacent muscle cell causes depolarization of the cell membrane - this triggers the action potential. At rest, the inner side of the cell membrane is negatively charged, and inside it contains more potassium ions than in the external environment, and less sodium . During the action potential, positively charged sodium ions enter the cell, reducing its negative charge (this process is called depolarization ). When a certain positive value of the charge of the inner side of the membrane is reached, potassium ions begin to leave the cell, gradually returning its membrane potential to the value characteristic of the resting state (this process is called ) [7] .
The start of muscle contraction begins with the release of acetylcholine near the motor end plate. Because of this, an action potential arises which is carried out at a speed of 2 m / s along the sarcolemma of the entire muscle fiber. Further, the action potential penetrates into the fiber through T-tubes [8] .
In the heart muscle, the action potential runs along the T-tube, causing the activation of L-type calcium channels, due to which calcium begins to enter the cell. The concentration of L-type calcium channels in T-tubes is higher than in the rest of the sarcolemma; therefore, most of the calcium ions enter the cell through T-tubes [9] . Inside the cell, calcium ions bind to ryanodine receptors , which are located on the membrane of the intracellular calcium depot - the sarcoplasmic reticulum. Activation of ryanodine receptors causes the release of calcium from the sarcoplasmic reticulum, which leads to a reduction in muscle cells [10] . In skeletal muscle, the L-type calcium channel is directly connected to the ryanodine receptor on the sarcoplasmic reticulum, due to which the ryanodine receptors are activated without an incoming calcium current [11] .
The importance of T-tubules is not limited to a high concentration of L-type calcium channels: they are able to synchronize the release of calcium in the cell. The rapid spread of the action potential over the network of T-tubes leads to the fact that L-type calcium channels are activated in them almost simultaneously. Since the sarcolemma very close to the sarcoplasmic reticulum in the region of the T-tubules, calcium release from the latter almost immediately starts. Due to the synchronization of calcium output, a stronger muscle contraction is achieved. In cells that do not have T-tubules, such as smooth muscle cells that have lost the functionality of cardiomyocytes or muscle cells that have artificially removed T-tubes, calcium entering the cell diffuses slowly in the cytoplasm and reaches the ryanodine receptors much more slowly, from -for which the muscle contracts less than in the presence of T-tubes [12] .
Since electromechanical conjugation occurs precisely in T-tubes, ion channels and other proteins necessary for this process are in much higher concentration in T-tubes than in the rest of the sarcolemma. This applies not only to L-type calcium channels, but also to β-adrenergic receptors [13] , and their stimulation enhances the release of calcium from the sarcoplasmic reticulum [14] .
Calcium Control
Since the inner space of T-tubules, in fact, is a continuation of the environment, the concentration of ions in it is about the same as in extracellular fluid. However, since the concentration of ions inside the T-tubes is very important (especially the concentration of calcium in the T-tubes of cardiomyocytes), it is necessary that these concentrations remain more or less constant. Due to the fact that the diameter of the T-tubes is very small, they capture ions. Due to this, with a decrease in the concentration of calcium in the environment ( hypocalcemia ), the concentration of calcium in the T-tubes does not change and remains sufficient to start the contraction [4] .
Through T-tubes, not only calcium enters the cell, but also its output from the cell. Due to this, the intracellular calcium concentration can be tightly controlled only in a small area, namely, in the space between the T-tube and the sarcoplasmic reticulum [15] . Sodium-calcium exchanger, as well as calcium ATPase are localized mainly in the membrane of T-tubes [4] . Sodium-calcium exchanger passively removes one calcium ion from the cell in exchange for the input of three sodium ions. Due to the fact that the process is passive, that is, it does not need energy in the form of ATP , calcium can enter and leave the cell through the exchanger depending on the combination of the relative concentration of Ca 2+ and Na + ions, as well as voltage on the cell membrane ( electrochemical gradient ). Calcium ATPase actively removes calcium from the cell, using ATP as an energy source [7] .
Detubulation
To study the functions of T-tubules, it is possible to artificially separate T-tubules and the cell membrane using a method known as detubulation. Glycerin [16] or formamide [12] is added to the extracellular fluid (for skeletal and cardiac muscles, respectively). These osmotically active agents cannot pass through the cell membrane, and when they are added to the extracellular fluid, the cells begin to lose water and contract. When these substances are removed, the cell quickly restores its volume and returns to its normal size, however, due to the rapid expansion of the cell, the T-tubules come off the cell membrane [17] .
Clinical Importance
In some diseases, the structure of T-tubules changes, which can lead to weakness of the heart muscle or a disturbance in the rhythm of its contraction. Violations in the structure of T-tubules can be expressed in the complete loss of these structures or only by a change in their orientation and branching method. Loss or violation in the structure of T-tubules often occurs with myocardial infarction [18] . A heart attack can lead to disturbances in the T-tubes in the ventricles, due to which the force of contraction decreases, as well as the chances of recovery [19] . Sometimes with a heart attack, there is an almost complete loss of T-tubules in the atria, which reduces atrial contractility and can cause atrial fibrillation [20] .
With structural changes in the T-tubes, L-type calcium channels may lose contact with the ryanodine receptors. As a result, the time required to increase the concentration of calcium increases, which leads to a weaker reduction and arrhythmias. However, violations in the T-tubes can be reversible, and it has been suggested that the structure of T-tubes can be restored to normal using interval training [4] [20] .
Study History
The idea of the existence of cell structures similar to T-tubules was first expressed in 1881. The time elapsed between the stimulation of the striated muscle cell and its contraction is too short to be due to the movement of the chemical signal from the sarcolemma to the sarcoplasmic reticulum. It has been suggested that such a short time may be associated with the presence of deep protrusions of the muscle cell membrane [21] [22] . In 1897, T-tubes were first seen using a light microscope in a cardiac muscle into which ink was previously injected. After the invention of the transmission electron microscope, the structure of T-tubes was studied in more detail [23] , and in 1971 the longitudinal components of the T-tube network were described [24] . In the 1990s and 2000s, using confocal microscopy, it was possible to obtain a spatial model of the network of T-tubules, as well as to determine their size and distribution [25] . With the discovery of , an association between T-tubules and calcium yield began to be traced [26] . For a long time, T-tubules were studied only on the example of skeletal muscles and ventricular heart muscle, but in 2009 it was possible to see a well-developed system of T-tubes in the muscle cells of the atria [20] . Current studies focus on the regulation of the structure of T-tubules and their changes in various cardiovascular diseases [27] .
Notes
- ↑ 1 2 3 Hong T. , Shaw RM Cardiac T-Tubule Microanatomy and Function. (English) // Physiological Reviews. - 2017 .-- January ( vol. 97 , no. 1 ). - P. 227-252 . - DOI : 10.1152 / physrev.00037.2015 . - PMID 27881552 .
- ↑ Haddock PS , Coetzee WA , Cho E. , Porter L. , Katoh H. , Bers DM , Jafri MS , Artman M. Subcellular Ca2 + i gradients during excitation-contraction coupling in newborn rabbit ventricular myocytes. (English) // Circulation Research. - 1999 .-- 3 September ( vol. 85 , no. 5 ). - P. 415-427 . - PMID 10473671 .
- ↑ Richards MA , Clarke JD , Saravanan P. , Voigt N. , Dobrev D. , Eisner DA , Trafford AW , Dibb KM Transverse tubules are a common feature in large mammalian atrial myocytes including human. (English) // American Journal of Physiology. Heart And Circulatory Physiology. - 2011 .-- November ( vol. 301 , no. 5 ). - P. 1996-2005 . - DOI : 10.1152 / ajpheart.00284.2011 . - PMID 21841013 .
- ↑ 1 2 3 4 5 Ibrahim M. , Gorelik J. , Yacoub MH , Terracciano CM The structure and function of cardiac t-tubules in health and disease. (English) // Proceedings. Biological Sciences. - 2011 .-- 22 September ( vol. 278 , no. 1719 ). - P. 2714-2723 . - DOI : 10.1098 / rspb.2011.0624 . - PMID 21697171 .
- ↑ 4. Calcium reuptake and relaxation. . www.bristol.ac.uk . Date of treatment February 21, 2017.
- ↑ Caldwell JL , Smith CE , Taylor RF , Kitmitto A. , Eisner DA , Dibb KM , Trafford AW Dependence of cardiac transverse tubules on the BAR domain protein amphiphysin II (BIN-1). (English) // Circulation Research. - 2014 .-- 5 December ( vol. 115 , no. 12 ). - P. 986–996 . - DOI : 10.1161 / CIRCRESAHA.116.303448 . - PMID 25332206 .
- ↑ 1 2 M., Bers, D. Excitation-contraction coupling and cardiac contractile force . - 2nd. - Dordrecht: Kluwer Academic Publishers, 2001 .-- ISBN 9780792371588 .
- ↑ Zilbernagl S., Despopoulos A. Visual physiology. - M .: BINOM. Laboratory of Knowledge, 2013 .-- S. 68. - 408 p. - ISBN 978-5-94774-385-2 .
- ↑ Scriven DR , Dan P. , Moore ED Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. (English) // Biophysical Journal. - 2000 .-- November ( vol. 79 , no. 5 ). - P. 2682-2691 . - DOI : 10.1016 / S0006-3495 (00) 76506-4 . - PMID 11053140 .
- ↑ Bers DM Cardiac excitation-contraction coupling. (Eng.) // Nature. - 2002 .-- 10 January ( vol. 415 , no. 6868 ). - P. 198-205 . - DOI : 10.1038 / 415198a . - PMID 11805843 .
- ↑ Rebbeck RT , Karunasekara Y. , Board PG , Beard NA , Casarotto MG , Dulhunty AF Skeletal muscle excitation-contraction coupling: who are the dancing partners? (Eng.) // The International Journal Of Biochemistry & Cell Biology. - 2014 .-- March ( vol. 48 ). - P. 28-38 . - DOI : 10.1016 / j.biocel.2013.12.001 . - PMID 24374102 .
- ↑ 1 2 Ferrantini C. , Coppini R. , Sacconi L. , Tosi B. , Zhang ML , Wang GL , de Vries E. , Hoppenbrouwers E. , Pavone F. , Cerbai E. , Tesi C. , Poggesi C. , ter Keurs HE Impact of detubulation on force and kinetics of cardiac muscle contraction. (Eng.) // The Journal Of General Physiology. - 2014 .-- June ( vol. 143 , no. 6 ). - P. 783-797 . - DOI : 10.1085 / jgp.201311125 . - PMID 24863933 .
- ↑ Laflamme MA , Becker PL G (s) and adenylyl cyclase in transverse tubules of heart: implications for cAMP-dependent signaling. (Eng.) // The American Journal Of Physiology. - 1999 .-- November ( vol. 277 , no. 5 Pt 2 ). - P. 1841-1848 . - PMID 10564138 .
- ↑ Bers DM Cardiac ryanodine receptor phosphorylation: target sites and functional consequences. (English) // The Biochemical journal. - 2006. - Vol. 396, no. 1 . - P. e1-3. - DOI : 10.1042 / BJ20060377 . - PMID 16626281 .
- ↑ Hinch R. , Greenstein JL , Tanskanen AJ , Xu L. , Winslow RL A simplified local control model of calcium-induced calcium release in cardiac ventricular myocytes. (English) // Biophysical Journal. - 2004 .-- December ( vol. 87 , no. 6 ). - P. 3723-3736 . - DOI : 10.1529 / biophysj.104.049973 . - PMID 15465866 .
- ↑ Fraser James a. , Hockaday Austin R. , Huang1 Christopher L.-H. , Skepper Jeremy N. [1] (Eng.) // Journal of Muscle Research and Cell Motility. - 1998. - Vol. 19 , no. 6 . - P. 613-629 . - ISSN 0142-4319 . - DOI : 10.1023 / A: 1005325013355 .
- ↑ Moench I. , Meekhof KE , Cheng LF , Lopatin AN Resolution of hyposmotic stress in isolated mouse ventricular myocytes causes sealing of t-tubules. (English) // Experimental Physiology. - 2013 .-- July ( vol. 98 , no. 7 ). - P. 1164-1177 . - DOI : 10.1113 / expphysiol.2013.072470 . - PMID 23585327 .
- ↑ Pinali C. , Malik N. , Davenport JB , Allan LJ , Murfitt L. , Iqbal MM , Boyett MR , Wright EJ , Walker R. , Zhang Y. , Dobryznski H. , Holt CM , Kitmitto A. Post-Myocardial Infarction T-tubules Form Enlarged Branched Structures With Dysregulation of Junctophilin-2 and Bridging Integrator 1 (BIN-1). (Eng.) // Journal Of The American Heart Association. - 2017 .-- May 4 ( vol. 6 , no. 5 ). - DOI : 10.1161 / JAHA.116.004834 . - PMID 28473402 .
- ↑ Seidel T. , Navankasattusas S. , Ahmad A. , Diakos NA , Xu WD , Tristani-Firouzi M. , Bonios MJ , Taleb I. , Li DY , Selzman CH , Drakos SG , Sachse FB Sheet-Like Remodeling of the Transverse Tubular System in Human Heart Failure Impairs Excitation-Contraction Coupling and Functional Recovery by Mechanical Unloading. (English) // Circulation. - 2017 .-- 25 April ( vol. 135 , no. 17 ). - P. 1632-1645 . - DOI : 10.1161 / CIRCULATIONAHA.116.024470 . - PMID 28073805 .
- ↑ 1 2 3 Dibb KM , Clarke JD , Horn MA , Richards MA , Graham HK , Eisner DA , Trafford AW Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. (English) // Circulation. Heart failure. - 2009 .-- September ( vol. 2 , no. 5 ). - P. 482-489 . - DOI : 10.1161 / CIRCHEARTFAILURE.109.852228 . - PMID 19808379 .
- ↑ Huxley AF The activation of striated muscle and its mechanical response. (Eng.) // Proceedings Of The Royal Society Of London. Series B, Biological Sciences. - 1971. - 15 June ( vol. 178 , no. 1050 ). - P. 1-27 . - PMID 4397265 .
- ↑ HILL AV. The abrupt transition from rest to activity in muscle. (Eng.) // Proceedings Of The Royal Society Of London. Series B, Biological Sciences. - 1949. - October ( vol. 136 , no. 884 ). - P. 399-420 . - PMID 18143369 .
- ↑ LINDNER E. Submicroscopic morphology of the cardiac muscle. (German) // Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie (Vienna, Austria: 1948). - 1957. - T. 45 , No. 6 . - S. 702-746 . - PMID 13456982 .
- ↑ Sperelakis N. , Rubio R. An orderly lattice of axial tubules which interconnect adjacent transverse tubules in guinea-pig ventricular myocardium. (Eng.) // Journal Of Molecular And Cellular Cardiology. - 1971. - August ( vol. 2 , no. 3 ). - P. 211-220 . - PMID 5117216 .
- ↑ Savio-Galimberti E. , Frank J. , Inoue M. , Goldhaber JI , Cannell MB , Bridge JH , Sachse FB Novel features of the rabbit transverse tubular system revealed by quantitative analysis of three-dimensional reconstructions from confocal images. (English) // Biophysical Journal. - 2008 .-- August ( vol. 95 , no. 4 ). - P. 2053-2062 . - DOI : 10.1529 / biophysj.108.130617 . - PMID 18487298 .
- ↑ Cheng H. , Lederer WJ , Cannell MB Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. (English) // Science (New York, NY). - 1993. - Vol. 262, no. 5134 . - P. 740-744. - PMID 8235594 .
- ↑ Eisner DA , Caldwell JL , Kistamás K. , Trafford AW Calcium and Excitation-Contraction Coupling in the Heart. (English) // Circulation Research. - 2017 .-- 7 July ( vol. 121 , no. 2 ). - P. 181-195 . - DOI : 10.1161 / CIRCRESAHA.117.310230 . - PMID 28684623 .