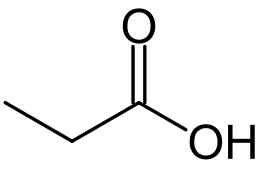
Propionic acid (propanoic acid, methylacetic acid, preservative E280) CH 3 CH 2 COOH is a colorless caustic liquid with a pungent odor. Propionic acid (from the Greek "protos" - first, "pion" - fat;) is named so because it is the smallest H (CH 2 ) n COOH acid, which exhibits the properties of fatty acids . Salts and anions of propionic acid are called propionates.
| Propionic acid | |
|---|---|
 | |
 | |
 | |
| Are common | |
| Systematic name | Propanoic acid |
| Traditional names | Propionic acid |
| Chem. formula | C 3 H 6 O 2 |
| Rat formula | CH 3 CH 2 COOH |
| Physical properties | |
| condition | Colorless liquid |
| Molar mass | 74.08 g / mol |
| Density | |
| Ionization energy | |
| Thermal properties | |
| T. melt. | −21 ° C |
| T. bale. | 141 ° C |
| T. aux. | 54 ° C |
| Etc. blast | |
| Steam pressure | |
| Chemical properties | |
| pK a | 4.88 |
| Structure | |
| Dipole moment | 0.63 D |
| Classification | |
| Reg. CAS number | 79-09-4 |
| PubChem | |
| Reg. EINECS number | |
| Smiles | |
| Inchi | |
| Codex Alimentarius | |
| RTECS | UE5950000 |
| Chebi | |
| ChemSpider | |
Content
Physical and chemical properties
Auto-ignition temperature 440 ° C.
Miscible with water (unlimited) and organic solvents.
By chemical properties - a typical representative of saturated carboxylic acids ; forms esters , amides , halides , etc.
History
Propionic acid was first described in 1844 by , who found it among sugar decomposition products. Over the next few years, other chemists received propionic acid in various ways, not realizing that they were receiving the same substance. In 1847, the French chemist Jean-Baptiste Dumas established that the acids obtained were one and the same substance, which he called propionic acid.
Getting
In nature, propionic acid is found in oil , formed during the fermentation of carbohydrates . In industry, it is obtained by ethylene carbonylation by the Reppe reaction ; catalytic oxidation of propionic aldehyde in the presence of cobalt or manganese ions ; as a by-product in the vapor-phase oxidation of C 4 —C 10 hydrocarbons. A large amount of propionic acid was previously obtained as a by-product in the production of acetic acid , but modern methods for producing acetic acid have made this method a secondary source of propionic acid.
Propionic acid is also obtained biologically by the metabolic decomposition of fatty acids containing an odd number of carbon atoms, and by the decomposition of certain amino acids. Propionibacterium bacteria produce propionic acid as the final product of their anaerobic metabolism . These bacteria are often found in the stomach of ruminants and in silage [2] , and partly because of their activity, Swiss cheese has its own flavor.
Derivatives
Propionates - salts and esters of propionic acid. Alkaline and alkaline earth salts of propionic acid are highly soluble in water and insoluble in organic solvents . Esters of propionic acid are poorly soluble in water, miscible with organic solvents.
Application
Propionic acid and its derivatives are used in the production of herbicides ( propanol , dichloroprol ), medicines ( ibuprofen , phenobolin , etc.), odoriferous substances (benzyl, phenyl, geranyl, linaloyl propionates), plastics (for example, polyvinyl propionate ), solvents (propyl, butyl, pentyl propionate, etc.), vinyl plasticizers and surfactants (glycol ethers).
Propionic acid inhibits the growth of mold and certain bacteria . Therefore, most of the propionic acid produced is used as a preservative in foods consumed by humans and in foods for animals. Propionic acid or its ammonium salt (ammonium propionate) is used directly in animal products. In foods consumed by people, especially in bread and other bakery products, propionic acid is used as sodium (sodium propionate ) or calcium (calcium propionate ) salt.
Security
The main danger of propionic acid is chemical burns, which can occur in contact with concentrated acid. In studies on laboratory animals, the only adverse effect associated with the long-term use of a small amount of propionic acid was the formation of ulcers in the esophagus and stomach due to the corrosive properties of the substance. In studies it was not found that propionic acid is toxic, mutagenic, carcinogenic and negatively affects the reproductive organs. In the body, propionic acid is rapidly oxidized, metabolized and excreted from the body as carbon dioxide in the Krebs cycle , without accumulating in the body.
Notes
- ↑ 1 2 3 4 http://www.cdc.gov/niosh/npg/npgd0529.html
- ↑ Romanov MN, Bato RV, Yokoyama MT, Rust SR PCR detection and 16S rRNA sequence-based phylogeny of a novel Propionibacterium acidipropionici applicable for enhanced fermentation of high moisture corn (English) // Journal of Applied Microbiology: Journal. - Oxford , UK : ; , 2004. - Vol. 97, no. 1 . - P. 38-47. - ISSN 1364-5072 . - DOI : 10.1111 / j.1365-2672.2004.02282.x . - PMID 15186440 . Archived March 15, 2015. (Retrieved March 15, 2015)
Literature
- Zefirov N.S., Kulov N.N. et al. Chemical Encyclopedia. - M .: Great Russian Encyclopedia, 1995. - T. 4. - P. 107-108. - ISBN 5-85270-092-4 .