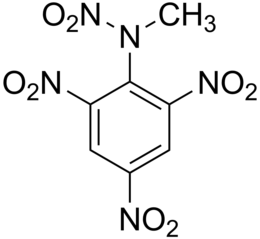
Tetrile - C 6 H 2 (NO 2 ) 3 N (CH 3 ) NO 2 - a powerful explosive , according to its explosive characteristics it belongs to secondary (blasting).
| Tetrile | |
|---|---|
 | |
 | |
| Are common | |
| Systematic name | 2,4,6-trinitrophenyl-N-methylnitramine |
| Traditional names | Tetril, nitramine, tetralit |
| Chem. formula | ( N O 2 ) 3 C 6 H 2 N (NO 2 ) CH 3 |
| Physical properties | |
| condition | solid |
| Molar mass | 287.15 g / mol |
| Density | |
| Thermal properties | |
| T. melt. | 129.5 ° C |
| T. decomp. | 187 ° C |
| Steam pressure | |
| Classification | |
| Reg. CAS number | |
| PubChem | |
| Reg. EINECS number | |
| Smiles | |
| Inchi | |
| RTECS | |
| Chebi | |
| UN number | |
| ChemSpider | |
Synonyms: 2,4,6-trinitro-N-methyl-N-nitroaniline; N-methyl-2,4,6-trinitrophenyl nitramine; methyl picrynitramine; N-methyl-N, 2,4,6-tetranitroaniline. Trade names: tetryl, nitramine, tetralite.
Content
Physico-chemical properties
The crystalline substance is white. But the color of the technical product is light yellow, due to impurities. It is soluble in acetone, concentrated nitric acid and benzene, worse in alcohol and dichloroethane . Practically insoluble in water. Non-hygroscopic. Reacts with alkalis and sodium and potassium carbonates . When heated in weak alkali solutions, it forms picrates . At the conc. (?), H2SO4 decomposes to methyl picramide. Reacts with aniline in benzene to form methylnitramine and 2-, 4-, 6-trinitrodiphenylamine. The pure product is resistant to weak acids and does not interact with ammonium nitrate . Due to the impurity of picric acid, the technical product corrodes ordinary steel and is incompatible with ammonium nitrate. When fused with TNT, it forms an adduct with t pl. 68 ° C. Due to the presence of nitro groups, it can form derivatives with carmine red metals, which are highly sensitive explosives with a flash point of 95-105 ° C. Toxic, with systematic contact, causes allergies or eczema on the skin, colors the skin red, requires special protective measures when outstanding. Chemical resistance is lower than that of TNT and some other nitro compounds, but sufficient for long-term storage under normal conditions. It is easily pressed to a high density (1.71 g / cm 3 at 2000 kgf / cm²). The crystalline density is 1.73 g / cm 3 , the usual charge density is 1.63 g / cm 3 Mohs hardness is less than 1.0.
In 100 cm 3 tetryl is dissolved in grams:
| Temperature | Water | Benzene | Acetone | Dichloroethylene | Alcohol | CCl4 | Ether | Carbon disulphide |
|---|---|---|---|---|---|---|---|---|
| 0 ° C | 0.005 | 3.45 | - | 1,5 | 0.32 | 0.007 | 0.188 | 0.009 |
| 17 ° C | 0.007 | - | - | - | 0.49 | 0.02 | - | 0.017 |
| 20 ° C | 0.008 | 9.99 | 45.82 | 3.8 | 0.56 | 0,025 | 0.418 | 0,021 |
| 30 ° C | 0.008 | - | - | - | 0.76 | 0,039 | 0.493 | 0,029 |
| 40 ° C | 0.011 | - | - | 7.7 | 1.12 | 0.058 | - | 0.056 |
| 45 ° C | 0.014 | - | - | - | 1.38 | 0,073 | - | 0,094 |
| 50 ° C | 0.019 | - | 111.85 | - | 1.72 | 0,095 | - | - |
| 60 ° C | 0,035 | - | - | 18.8 | 2.64 | 0.154 | - | - |
| 70 ° C | 0,053 | 21.86 | - | - | 4.23 | 0.241 | - | - |
| 75 ° C | 0,066 | - | - | - | 5.33 | 0.247 | - | - |
| 80 ° C | 0,081 | 42,43 | - | 64.5 | - | - | - | - |
| 100 ° C | 0.184 | - | - | - | - | - | - | - |
Susceptibility to heat and external influences
Melting point - 129.5 ° C with decomposition (technical melts at 128.8 ° C). Thermally stable up to 100 ° C. t aux. (?) - OK. 190 ° С (for TNT - 290 ° С, for TNF - 310 ° С). At 190 ° C, it quickly burns out with a bright bright flame with noise. More powerful and sensitive explosive than TNT or picric acid.
Chemical resistance: a pure product withstands the Eble test at 80 ° C for not more than 50 minutes This instability is explained by the admixture of tetranitrophenylmethyl nitramine (m-nitrotetrile), which is formed from monomethylaniline contained in dimethylaniline and is already hydrolyzed by boiling water to acid trinitrophenylmethyl nitraminophenol and nitrous acid.
Sensitivity to shock: for a load of 2.5 kg (50% probability of detonation) - 37 cm, ( RDX - 28 cm, TNT - 148 cm). For a load of 10 kg (height 25 cm), a 50-60% probability of an explosion (TNT - 4-8%, RDX - 70-80%).
Susceptibility to detonation: 0.29 g for explosive mercury , 0.09 g for TA , 0.05 g for HMTD or 0.03 g for lead azide .
Explosive characteristics
- Explosion heat: 4.6 MJ / kg (960 kcal / kg at 0.98 g / cm 3 , 1160 kcal / kg at 1.69 g / cm 3 ).
- Heat of formation: +7.6 kcal / mol.
- Enthalpy of formation: +16.7 kcal / kg.
- Explosion temperature: 2950 Kelvin (approximately 2676 ° C).
- Knock speed: 7500 m / s at a density of 1.63 g / cm 3 . At 1.70 g / cm 3, the detonation velocity is 7620 m / s and the pressure at the detonation wave front is 25 GPa, (TNT 19 GPa, RDX 34.7 GPa ( at 1.6 and 1.8 g / cm 3 , respectively )).
- Brisance: 113-123% (54-59 g) of TNT (48 g) in a sand sample. Compression of lead columns - 19 mm (thickness - 16.5 mm).
- Discharge in Pb-block : 390 ml for chemically pure tetrile, and 340 ml for technical product (the technical product contains low melting impurities and has lower energy). For comparison: TNT - 285 ml, TEN - 500 ml.
- Performance in a ballistic mortar: 126-132% of TNT.
- The volume of explosion products: 765 l / kg.
Getting
Tetrile is usually prepared by nitration of dimethylaniline . When dimethylaniline is added to conc. nitric acid self-ignition occurs. In industry, tetryl is obtained by dissolving dimethylaniline in excess of 92-96% sulfuric acid (usually 1 hour of dimethylaniline for 8-14 hours of 96% sulfuric acid) and nitrating the resulting solution of dimethylaniline sulfate conc. nitric acid or melange. The reaction is accompanied by the oxidation of one methyl group and a large heat release. Throughout the process, the course of the reaction and temperature should be carefully monitored, otherwise it is possible to resinize dimethylaniline or even an outbreak. In Germany during World War II, tetrile was made from dinitrochlorobenzene, treated with an aqueous solution of methylamine and nitrated with dinitromethylaniline nitrating mixture to tetryl. This method was safer and allows the use of dinitrochlorobenzene, a widely available raw material.
Very pure tetryl can be obtained by nitration of dimethylaniline with a large excess of nitric acid with a density of 1.4 (65%). For this, 1 part of dimethylaniline is dissolved in 40 parts of nitric acid at a temperature of up to 7 ° C. Then the temperature is carefully raised to 60 ° C, and upon completion of the relatively violent oxidation reaction, it is heated to 90 ° C. Upon cooling, tetrile is released with a 78% yield.
Tetrile can also be obtained by two-stage nitration of monomethylaniline with nitric acid. In the first stage, monomethylaniline is nitrated with 50-60% nitric acid to dinitromethylaniline, and in the second to tetryl.
Application
It was first obtained in 1877. It began to be used as a secondary charge in detonator capsules and other means of detonation from 1906 in Germany. In Russia and England - since 1910. During the 2nd World War, in addition to detonating means, it was also used in mixed explosives to equip ammunition, for example, in small-caliber shells mixed with a phlegmatizer and in alloys with TNT (the so-called. Tetritols, which were used individually and as a fusible base for injection mixtures with RDX. Tetriols with a high content of tetryl are equivalent to 50/50 pentolite in cumulative ammunition effectiveness). Currently, tetrile is of secondary importance and is discontinued in most countries (for example, in the USA), and more powerful heating elements, and especially hexogen, are used instead.
Links
Notes
Literature
- Zefirov N.S. et al. 4 Half-Three // Chemical Encyclopedia. - M .: Big Russian Encyclopedia, 1995 .-- 639 p. - 20,000 copies. - ISBN 5-85270-092-4 .
- Volkov I. Subversive devices at the device of barriers. - Moscow: State Military Publishing House, 1933.