Chloropicrin (trichloronitromethane) CCl 3 NO 2 - liquid with a pungent irritating odor; a technical product may have different shades of odor, depending on the purity and method of preparation. It is used as a component of fumigant mixtures in agriculture [2] , in gas smoking chambers for checking the tightness of insulating and filtering gas masks.
| Chloropicrin | |
|---|---|
 | |
 | |
| Are common | |
| Systematic name | Chloropicrin, nitrochloroform, trichloronitromethane |
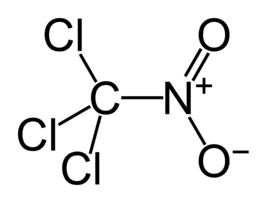
| Chem. formula | CCl 3 NO 2 |
| Physical properties | |
| condition | liquid |
| Molar mass | 164.376 g / mol |
| Density | 1.6539 (+20 ° C) |
| Thermal properties | |
| T. melt. | −69 ° C |
| T. bale. | 112.3 ° C |
| Steam pressure | |
| Classification | |
| Reg. CAS number | |
| PubChem | |
| Reg. EINECS number | |
| Smiles | |
| Inchi | |
| RTECS | |
| Chebi | |
| UN number | |
| ChemSpider | |
| Security | |
| Toxicity | highly toxic, irritant, lacrimator  |
| NFPA 704 |  0 four 3 |
Synthesis Methods
Chloropicrin was first obtained by the Scottish chemist John Stenhouse back in 1848 when 2,4,6-trinitrophenol ( picric acid ) was reacted with bleach . Later, this ancient process was used in industrial processes by which chloropicrin was obtained for military purposes during the First World War . The action of calcium hypochlorites (that is, cheap bleach) leads to a cleavage of the 2,4,6-trinitrophenol cycle and, at the same time, to chlorination of the cleavage products.
Instead of sparingly soluble picric acid, soluble calcium picrate was used, which was prepared by mixing picric acid with calcium oxide, and a slurry of bleach was added to the mixture. The heat of this reaction is sufficient to evaporate the resulting chloropicrin, the vapor of which is then condensed. Then, chloropicrin is easily distilled with steam for purification.
In the reaction, not only bleach can be used, but other hypochlorites, including those formed in situ when chlorine is passed into an alkaline solution of picric acid or other nitrophenols.
Currently, the main industrial method for the synthesis of chloropicrin is the chlorination of nitromethane in an alkaline environment:
Chloropicrin can also be synthesized by nitration of chloroform with acetyl nitrate:
Physico-chemical properties
Chloropicrin is a colorless, oily, highly light-refracting liquid with a sharp specific odor. In the light, it becomes greenish-yellowish, which can be explained by its partial decomposition with the formation of chlorine and nitrogen oxides. The boiling point is 112-113 ° C (760 mmHg) and +49 ° C (40 mmHg), the melting point is +62.2 (+64) ° C.
Dependence of steam pressure, maximum concentration on pressure.
| Temperature ° C | Vapor pressure, mmHg Art. | The maximum concentration, mg / l |
|---|---|---|
| 0 | 5.91 | 57 |
| ten | 10.87 | 104 |
| 20 | 16.91 | 184 |
| thirty | 30.50 | 295 |
The wide temperature range in which chloropicrin exists in the liquid state, and its high volatility even at low temperatures, allows it to be used at any time of the year. In the winter months, a concentration is achieved that is less than absolutely toxic, but sufficient to suppress the enemy.
Persistence in an area devoid of vegetation is at ordinary temperature about 6 hours. During this period, access to the infected area or its overcoming without a gas mask is impossible. Solubility in water at +25 ° C, is 0.16%. Chloropicrin is highly soluble in organic solvents.
Of inorganic solvents, it dissolves well in silicon tetrachloride and tin. It should be noted that good miscibility with OM , for example, mustard gas, mustard nitrogen, diphosgene, phosgene, organophosphorus OM, makes chloropicrin an important component for tactical mixtures, especially in the case of relatively low melting OM, which makes it possible to use them in winter time.
Chloropicrin is not hydrolyzed with water. Chloropicrin is easily degassed with an alcoholic solution of sodium sulfide, or with hydrazine solutions.
Chloropicrin under high pressure and heating turns into phosgene .
Toxic Properties
Chloropicrin irritates the skin and mucous membranes. It causes lacrimation, closing of the eyelids, bronchitis and pulmonary edema. In the affected person, the strongest uncontrolled convulsions of the muscles of the diaphragm begin. Liquid chloropicrin causes severe skin lesions.
In most people, a concentration of 0.002 mg / l for 3-30 s causes lacrimation and closure of the eyelids, a concentration of 0.05 mg / l is intolerable. Higher concentrations lead to stomach pain, vomiting and loss of consciousness. A concentration of about 0.2 mg / l in a few seconds or minutes leads to a complete loss of combat readiness. The danger of chloropicrin is manifested in the fact that, to a certain concentration limit, it is perceived by the smell simply as a substance that smells of mustard that smells moderately, the symptoms of damage described above occur suddenly.
Respiratory damage occurs at concentrations above 0.1 mg / L. As a lethal concentration indicate 2 mg / l with an exposure of 10 minutes. With this concentration, death occurs within a few minutes.
Chloropicrin as a chemical warfare agent (OM)
Vapors of chloropicrin have a strong tear, and in high concentrations they have a choking and poisonous effect. In this regard, chloropicrin was used to a limited extent in the First World War as a chemical warfare agent (for example, against the Russian army in the summer of 1915 ), and also as a solvent for the combat use of other BWA (for the first time in the middle of 1916 by the German army mixed with diphosgene ) .
During the suppression of the anti-Bolshevik uprising in the Tambov province , three cases of the use of shells of the AFL type with chloropicrin for smoking rebels from the forest were recorded, which did not lead to noticeable results [3] .
Due to the low toxicity of modern OM and the strong irritating effect, chloropicrin is not considered as a combat OM. With other OM, chloropicrin is also not currently used, since its irritating effect would almost immediately make it possible to detect the use of OM.
Due to the annoying effect and ease of handling, chloropicrin is used as a training (simulation of gas attack ) and verification (testing of chemical protection) OM. To do this, in an army tent, a moderate amount of chloropicrin, about 50 ml, is heated in a water bath. However, due to the fact that when heated, chloropicrin decomposes with the formation of phosgene , its use as a training agent requires precautionary measures. In this regard, at present, chloropicrin as a training agent is gradually being replaced by other irritant agents.
Links
- Chloropicrin // PubChem Compound
Notes
- ↑ http://www.cdc.gov/niosh/npg/npgd0132.html
- ↑ Whitacre, David M. Reviews of Environmental Contamination and Toxicology /. - Springer, 2009-08-19. - ISBN 1441900276 , 9781441900272.
- ↑ Aleksandr V. Glushko, Natal'ya I. Shilo “Clear the Woods the Thugs are Hiding in With Poisonous, Asphyxiating, Gases ...”: Myths and Facts about the Tambov Uprising Voennyi Sbornik, 2013, No. 2, pp. 103-125.
