Radiocarbon analysis is a type of radioisotope dating used to determine the age of biological remains, objects and materials of biological origin by measuring the content of a 14 C radioactive isotope in a material with respect to stable carbon isotopes . Proposed by Willard Libby in 1946 ( Nobel Prize in Chemistry , 1960 ).
Physical grounds

2: Decay 14 C.
3: The equilibrium condition for living organisms and the disequilibrium for dead organisms in which radiocarbon decays without replenishment from the outside
Carbon , which is one of the main components of biological organisms, is present in the earth's atmosphere in the form of stable isotopes 12 C (98.89%) and 13 C (1.11%) and radioactive 14 C, which is present in trace amounts (about 10 −10 %). The 14 C isotope is constantly formed mainly in the upper atmosphere at an altitude of 12-15 km in the collision of secondary neutrons from cosmic rays with atmospheric nitrogen nuclei:
On average, about 7.5 kg of radiocarbon is formed in the Earth’s atmosphere per year with a total of ~ 75 tons .
The formation of radiocarbon due to natural radioactivity on the Earth's surface is negligible.
The radioisotope of carbon 14 C is susceptible to β - decay with a half-life T 1/2 = 5730 ± 40 years [1] , decay constant λ = 1.209 · 10 −4 year −1 :
The ratio of radioactive and stable carbon isotopes in the atmosphere and in the biosphere is approximately the same due to active mixing of the atmosphere, since all living organisms constantly participate in carbon metabolism, receiving carbon from the environment, and isotopes, due to their chemical indistinguishability, participate in biochemical processes practically in the same way.
The specific carbon activity in living organisms exchanging carbon with an atmospheric reservoir corresponds to the atmospheric content of radiocarbon and amounts to 13.56 ± 0.07 decay per minute per gram of carbon. With the death of the body, carbon metabolism ceases. After that, stable isotopes are preserved, and the radioactive ( 14 C) gradually decays, as a result, its content in the remains gradually decreases. Knowing the initial ratio of the isotope content in the body and determining their current ratio in the biological material by the mass spectrometric method or measuring the activity by dosimetry methods, it is possible to establish the time elapsed since the death of the body.
Application
To determine the age, carbon is emitted from the fragment of the test sample (by burning a previously purified fragment), the radioactivity is measured for the extracted carbon, based on this, the isotope ratio is determined, which shows the age of the samples. A carbon sample for measuring activity is usually injected into a gas that fills a proportional counter , or into a liquid scintillator . Recently, for very low contents of 14 C and / or very small masses of samples (several mg), accelerator mass spectrometry has been used to directly determine the content of 14 C. For 2010, the maximum age of a sample that can be precisely determined by the radiocarbon method is about 60 000 years, that is, about 10 half-lives of 14 C. During this time, the content of 14 C decreases by about 1000 times (about 1 decay per hour per gram of carbon).
Measurement of the age of an object by a radiocarbon method is possible only when the ratio of isotopes in the sample has not been violated during its existence, that is, the sample was not contaminated with carbon-containing materials of a later or earlier origin, radioactive substances and was not exposed to strong radiation sources. Determining the age of such contaminated samples can make huge mistakes. Over the past decades since the development of the method, a great deal of experience has been gained in detecting contaminants and in cleaning samples from them. For dating, the least contaminated components are isolated from samples by chemical methods. Cellulose is used in radiocarbon analysis of plant residues, and collagen is secreted when dating bones, horns, and other animal residues. The error of the method currently ranges from seventy to three hundred years.
One of the most famous cases of using the radiocarbon method is the study of fragments of the Shroud of Turin , conducted in 1988 , simultaneously in several laboratories using the blind method . Radiocarbon analysis made it possible to date the shroud from the 11th to 13th century . Skeptics consider this result a confirmation that the shroud is a medieval fake. Supporters of the authenticity of the relics consider the data obtained as a result of contamination of the shroud with carbon during a fire and subsequent washing in boiling oil in the 16th century.
Calibration
Libby's initial assumptions on which the method of radiocarbon dating is based are that the ratio of carbon isotopes in the atmosphere in time and space does not change, and the content of isotopes in living organisms exactly corresponds to the current state of the atmosphere. However, as it was established later, these assumptions are true only approximately. The content of the 14 C isotope in the atmosphere depends on many factors, such as:
- cosmic ray intensity and solar activity;
- latitude of the terrain;
- state of the atmosphere and magnetosphere;
- volcanic activity (carbon contained in volcanic emissions, “ancient”, practically free of 14 C);
- carbon cycle in nature;
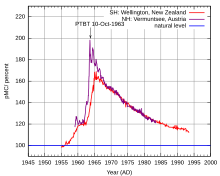
- conducting atmospheric nuclear tests that created in the 1950s and 60s a significant emission (about 0.5 tons) of radiocarbon into the atmosphere ( bomb effect );
- the burning of a large number of fossil fuels (the carbon contained in oil, natural gas and coal is “ancient”, practically not containing 14 C) - the so-called Suess effect that arose with the beginning of the industrial revolution in the 19th century.
The last two factors make it impossible to conduct accurate radiocarbon dating of 20th century samples.
In addition, studies have shown that due to the difference in the atomic masses of carbon isotopes, chemical reactions and processes in living organisms proceed at slightly different rates, which violates the natural ratio of isotopes (the so-called isotope fractionation effect) [4] . Another important effect ( reservoir effect ) - the delayed achievement of radiocarbon equilibrium in the World Ocean due to its slow [5] exchange of carbon with the atmospheric reservoir - leads, if not taken into account, to an apparent increase in the age of the remains of marine organisms, as well as those of land organisms whose diet consisted mainly of sea food. An understanding of the processes associated with the carbon exchange in nature and the influence of these processes on the ratio of isotopes in biological objects was not reached immediately. Thus, the use of the radiocarbon method without taking into account these effects and their amendments can generate significant errors (of the order of a millennium), which often occurred in the early stages of the development of the method, until the 1970s.
At present, for the correct application of the method, a thorough calibration has been carried out, taking into account the change in the ratio of isotopes for different eras and geographical regions, as well as the specifics of the accumulation of radioactive isotopes in living things and plants. To calibrate the method, it is used to determine the ratio of isotopes for objects whose absolute dating is known. One of the sources of calibration data is dendrochronology . Comparisons were also made of determining the age of samples by the radiocarbon method with the results of other isotopic dating methods. Now IntCal is used as a standard calibration curve, the first version of which was published in 1998 (see. Fig.) [2] . The following updated versions of the calibration curve used to convert the measured radiocarbon age of the sample to absolute age were published in 2004, 2009 [6] and 2013 [3] . The IntCal13 calibration curve was plotted separately for the northern and southern (SHCal13) hemispheres, it covers the last 50,000 years and was obtained on the basis of thousands of measurements of precisely dated tree rings (last 12,000 years), annual coral increments and foraminifera deposits. Comparison of sediments at the bottom of the Japanese lake Suigetsu for the period from 12,000 to 40 thousand years ago with the information obtained by dendrochronologists in the analysis of tree rings led to amendments that shifted the data by 300-400 years into the past [7] [8] . Calibration for marine objects is performed according to a separate curve Marine13, since the rate of carbon exchange in the marine reservoir is slower than atmospheric.
It can be stated that in its modern form on the historical interval (from tens of years to 60-70 thousand years into the past), the radiocarbon method can be considered a fairly reliable and qualitatively calibrated independent method for dating objects of biological origin.
Criticism of the method
Despite the fact that radiocarbon dating has long been included in scientific practice and is widely used, there is criticism of this method in semi-scientific publications and on the Internet, casting doubt on the validity of its use for dating of historical artifacts (especially of a later period). As a rule, the radiocarbon method is criticized by supporters of the “ scientific creationism ” of the “ New Chronology ” and other pseudoscientific concepts. Some examples of objections to radiocarbon dating are given in the Critique of Natural Science Methods section in Fomenko’s New Chronology . Typically, such a criticism of radiocarbon analysis is based on the earliest scientific publications reflecting the state of the methodology in the 1960s and on a misunderstanding of the fundamentals of the method and features of calibration [9] .
Fossil Carbon Impact
In 2015, H. Graven ( Imperial College London ) calculated [10] that further burning of fossil fuels at an existing pace due to the emission of “ancient” carbon into the atmosphere will lead to the indistinguishability of modern samples from older ones by the radiocarbon method [11] [12 ] [12 ] ] (although samples that arose prior to industrialization and do not exchange carbon with the atmosphere, this effect, of course, does not affect). Currently, the emission of fossil carbon into the atmosphere leads to the apparent “aging” of atmospheric carbon by about 30 years per year [10] .
See also
- Optical dating
- Thermoluminescent dating
Literature
- Gerasimov I.P. Radiocarbon studies of the Radiometric Laboratory of the Institute of Geography, USSR Academy of Sciences: Communication. 1-5: // Bulletin of the Commission for the Study of the Quaternary . Messaging 1: 1975. No. 44. S. 154-159; Messaging 2: 1976. No. 46. S. 185-189; Messaging 3: 1979. No. 49. S. 179-187; Messaging 4: 1980. No. 50. S. 206-213; Messaging 5: 1983. No. 52. P. 205-211.
- Wagner G. A. Scientific dating methods in geology, archeology and history: Textbook. - M .: Technosphere, 2006 .-- 534 p. - ISBN 5-94836-037-7 .
- Koronovsky N.V. General Geology: Textbook. - 2nd ed. - M .: Publishing house "KDU", 2010. - S. 122-124. - 526 p. - ISBN 978-5-98227-682-7 .
- * L. Currie "The Remarkable Metrological History of Radiocarbon Dating II . " J. Res. Natl. Inst. Stand. Technol. 109 (2004) 185-217.
Notes
- ↑ Godwin, H. Half-life of radiocarbon (Eng.) // Nature. - 1962. - Vol. 195 , no. 4845 . - P. 984 . - DOI : 10.1038 / 195984a0 . - .
- ↑ 1 2 Stuiver M., Reimer PJ, Braziunas TF High-precision radiocarbon age calibration for terrestrial and marine samples (Eng.) // Radiocarbon: journal. - 1998. - Vol. 40 . - P. 1127-1151 .
- ↑ 1 2 Reimer PJ et al. IntCal13 and Marine13 Radiocarbon Age Calibration Curves 0-50,000 Years cal BP // Radiocarbon: journal. - 2013. - Vol. 55 . - P. 1869-1887 . - DOI : 10.2458 / azu_js_rc.55.16947 .
- ↑ G.A. Wagner , p. 164.
- ↑ The characteristic time of carbon homogenization in the oceans is about 1000 years.
- ↑ IntCal09 Supplemental Data
- ↑ New chronology from Suigetsu
- ↑ Bronk Ramsey C. et al. A Complete Terrestrial Radiocarbon Record for 11.2 to 52.8 kyr BP (Eng.) // Science. - Vol. 338. - Iss. 6105 . - P. 370-374. - DOI : 10.1126 / science.1226660 .
- ↑ Levchenko V. On “radiocarbon through the eyes of Fomenko” and the “scientific” foundations of the New Chronology: polemic notes
- ↑ 1 2 Graven Heather D. Impact of fossil fuel emissions on atmospheric radiocarbon and various applications of radiocarbon over this century // Proceedings of the National Academy of Sciences. - 2015. - July 20 ( t. 112 , No. 31 ). - S. 9542-9545 . - ISSN 0027-8424 . - DOI : 10.1073 / pnas . 1504467112 .
- ↑ [1] .
- ↑ [2] .
Links
- E.N. Chernykh, N. B. Chernykh. Dendrochronology and radiocarbon dating in archeology
- Sampling procedure for radiocarbon and dendrochronological analysis. Tutorial
- V. Levchenko. Radiocarbon and absolute chronology: notes on the topic.
- V.A. Dergachev. Radiocarbon Chronometer Mirror .
- A new method for radiocarbon dating of artifacts does not destroy
- OxCal radiocarbon calibration program
- B. F. Khasanov. About the radiocarbon dating method