SNARE (from the English soluble NSF attachment receptor ) is a large group of proteins that fuse intracellular transport vesicles with a cell membrane ( exocytosis ) or a target organelle, such as a lysosome . About 60 SNARE proteins are counted. The proteins of the group are divided into two functional categories: vesicular proteins ( v-SNARE ) and proteins of the host organelle ( t-SNARE ). A new structural classification divides the group into R-SNARE and Q-SNARE . The most studied proteins are those that deliver synaptic vesicles to the presynaptic membrane and their fusion. These proteins are targets of the dangerous bacterial toxins of botulism and tetanus .
Structure
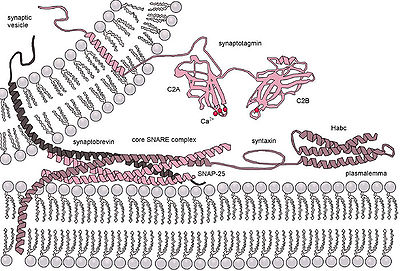
SNARE proteins are small but widely represented membrane proteins . Despite the large difference in structure and size, these proteins are united by the presence of the same cytosolic domain of 60–70 amino acids , called the SNARE motif (or SNARE domain), capable of forming a reversible but strong four-helix linkage. Such a metastable trans- SNARE complex includes syntaxin 1 and SNAP-25 located on the cell membrane, and synaptobrevins on the surface of the delivered presynaptic vesicle. Syntaxin and synaptobrevins are connected to the membrane with a C- terminal fragment and provide one alpha-helix in the four-helix linkage of the complex. SNAP-25 is anchored on the membrane due to palmitoyl acyl chains and provides the two remaining alpha helices.
Links
- Lang T., Jahn R. Core proteins of the secretory machinery (neopr.) // Handb Exp Pharmacol. - 2008. - No. 184 . - S. 107-127 . - DOI : 10.1007 / 978-3-540-74805-2_5 . - PMID 18064413 .
- Brunger AT, Jin R., Breidenbach MA Highly specific interactions between botulinum neurotoxins and synaptic vesicle proteins (English) // Cell. Mol. Life Sci. : journal. - 2008. - April. - DOI : 10.1007 / s00018-008-8088-0 . - PMID 18425411 .
See also
- Exocytosis