Copper sulfate (II) ( sulfate, copper sulfate ) is an inorganic compound , a copper salt of sulfuric acid with the formula Cu S O 4 . Non-volatile substance, odorless. In anhydrous form - a white powder, very hygroscopic. In the form of crystalline hydrates, transparent non-hygroscopic crystals of various shades of blue with a bitter-metal astringent taste, gradually disappear in the air (they lose crystallization water).
| Copper sulphate | |
|---|---|
 | |
 | |
| Are common | |
| Systematic name | copper (II) sulfate |
| Traditional names | pentahydrate: copper sulfate |
| Chem. formula | Cu S O 4 |
| Physical properties | |
| condition | crystalline |
| Molar mass | 159,609 (sulfate) 249.685 (pentahydrate) g / mol |
| Density | 3.64 g / cm³ |
| Hardness | 2.5 [1] |
| Thermal properties | |
| T. decomp. | above 650 ° C |
| Chemical properties | |
| pK a | 5⋅10 −3 |
| Structure | |
| Coordination geometry | Octahedral |
| Crystal structure | bezv. - rhombic pentahydrate - triclinic pinacoid trihydrate - monoclinic |
| Classification | |
| Reg. CAS number | 7758-98-7 |
| PubChem | |
| Reg. EINECS number | |
| Smiles | |
| Inchi | |
| Codex Alimentarius | |
| RTECS | GL8800000 |
| Chebi | |
| ChemSpider | |
| Security | |
| MPC | in the air: mp 0.009, ss 0.004; in water: 0.001 |
| LD 50 | rats, oral [2] : 612.9 mg / kg mouse, oral: 87 mg / kg |
| Toxicity | Moderately toxic, irritant, hazardous to the environment.  |
| NFPA 704 |  0 2 one |
Copper (II) sulfate is highly soluble in water. From aqueous solutions crystallized blue pentahydrate CuSO 4 · 5H 2 O - copper sulphate . The toxicity of copper sulfate for warm-blooded animals is relatively low, at the same time, it is highly toxic for fish.
It has disinfectant , antiseptic , astringent properties. It is used in medicine, in crop production as an antiseptic, fungicide or copper-sulfur fertilizer .
The hydration reaction of anhydrous copper (II) sulfate is exothermic and proceeds with significant heat release.
Content
Being in nature
It is found in nature in the form of minerals chalcanthite (CuSO 4 · 5H 2 O), chalcocyanite (CuSO 4 ), bonattite (CuSO 4 · 3H 2 O), butite (CuSO 4 · 7H 2 O) and as part of some other minerals [3] .
Getting
In Industry
In industry, contaminated copper (II) sulfate is obtained by dissolving copper and copper wastes in dilute sulfuric acid H 2 SO 4 by blowing air:
dissolution of copper (II) oxide CuO in H 2 SO 4 :
sulfatizing roasting of copper sulfides and as a by-product of electrolytic refining of copper.
In the laboratory
In the laboratory, CuSO 4 can be obtained by the action of concentrated sulfuric acid on copper when heated:
the temperature should not exceed 60 ° C, at a higher temperature in significant quantities, a by-product is formed - copper (I) sulfide :
Also, in laboratory conditions, copper (II) sulfate can be obtained by neutralizing copper (II) hydroxide with sulfuric acid; accordingly, pure reagents are used to obtain high-purity copper sulfate:
Pure copper sulfate can be obtained as follows. 120 ml of distilled water are poured into a porcelain cup, 46 ml of chemically pure sulfuric acid with a density of 1.8 g / cm 3 are added and 40 g of pure copper (for example, electrolytic) are added to the mixture. Then it is heated to 70–80 ° С and at this temperature gradually over an hour, in 1 ml portions, 11 ml of conc. nitric acid . If the copper is covered with crystals, add 10-20 ml of water. When the reaction is completed (the evolution of gas bubbles ceases), the copper residues are removed and the solution is evaporated until crystals appear on the surface of the film and allowed to cool. The precipitated crystals should be recrystallized 2-3 from distilled water and dried [4] .
Cleaning
It is possible to purify contaminated or technical copper sulfate by recrystallization - the substance is dissolved in boiling distilled water until the solution is saturated, and then it is cooled to approximately +5 ° C. The resulting crystal precipitate is filtered off. However, even repeated recrystallization does not allow to get rid of the impurity of iron compounds, which are the most common impurity in copper sulfate.
For complete purification, copper sulfate is boiled with lead dioxide PbO 2 or barium peroxide BaO 2 until a filtered sample of the solution shows the absence of iron. Then the solution is filtered and evaporated until crystals appear on the surface of the film, after which it is cooled to crystallize [4] .
According to N. Shoorl, copper sulfate can be purified as follows: add small amounts of hydrogen peroxide H 2 O 2 and sodium hydroxide NaOH to a hot CuSO 4 solution , boil and filter the precipitate. Crystals precipitated from the filtrate undergo recrystallization twice. The resulting substance has a purity not lower than the qualification "ChP" [4] .
Deep Cleaning
There is a more complex method of purification, which allows to obtain high purity copper sulfate with an impurity content of about 2 · 10 -4 %.
For this, an aqueous solution of copper sulfate saturated at 20 ° C is prepared (only bidistilled water is used). Hydrogen peroxide is added to it in an amount of 2-3 ml of a 30% solution per 1 liter, mixed, freshly precipitated basic copper carbonate is added in an amount of 3-5 grams, heated and boiled for 10 minutes to decompose H 2 O 2 .
Then the solution is cooled to 30–35 ° C, filtered and poured 15 ml of a 3% solution of sodium diethyldithiocarbamate and kept in a mixer for three to four hours without lowering the temperature. Then the solution is quickly filtered from large flakes of the complexes and BAU-A activated carbon is introduced for half an hour with stirring. Then the solution should be filtered by vacuum .
Then, about 200 ml of a saturated grade “Na” NaCl solution is poured into 1 CuSO 4 solution and pure aluminum is introduced into the wire or scraps until the reaction is complete, copper is released and the solution becomes clear ( hydrogen is released ). Separated copper is separated from aluminum by shaking, the precipitate is washed by decantation, first with water, then it is poured with a hot 5-10% hydrochloric acid solution of ChP during shaking for one hour and constant heating to 70–80 ° С, then it is washed with water and filled with 10-15% sulfuric acid (OSH 20-4) for an hour with heating at the same temperature range. The purity of further products depends on the degree and thoroughness of washing with acids, as well as the qualification of the reagents used below.
After washing with acids, copper is again washed with water and dissolved in 15-20% sulfuric acid (OSH 20-4) without its large excess with the addition of hydrogen peroxide (OSH 15-3). After completion of the reaction, the resulting acidic solution of copper sulfate is boiled to decompose the excess peroxide and neutralized until the initially precipitated precipitate is completely dissolved by distilled 25% ammonia solution (OSH 25-5) or a solution of ammonium carbonate purified by the complex adsorption method to very pure is added.
After standing for a day, the solution is slowly filtered. Sulfuric acid (TSP) is added to the filtrate until a bluish-green precipitate completely precipitates and is kept until enlargement and transition to green basic copper sulfate . The green precipitate is left to compact and washed thoroughly with water until complete removal of soluble impurities. Then the precipitate is dissolved in sulfuric acid, filtered, adjusted to pH = 2.5-3.0 and recrystallized twice with rapid cooling, and upon cooling, the solution is stirred each time to obtain smaller crystals of copper sulfate. The precipitated crystals are transferred to a Buchner funnel and the residual mother liquor is removed using a water-jet pump . The third crystallization is carried out without acidifying the solution to obtain slightly larger and more shaped crystals [5] .
Physical Properties
Copper (II) sulfate pentahydrate (vitriol) - blue transparent crystals of triclinic syngony. The density of 2.284 g / cm 3 . At a temperature of 110 ° С, 4 water molecules are split off, at 150 ° С, complete dehydration occurs [6] .
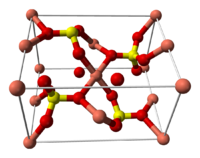
Crystalline Structure
The structure of copper sulfate is shown in the figure. As can be seen, two SO 4 2− anions and four water molecules (in the plane) are coordinated around the copper ion, and the fifth water molecule plays the role of bridges that combine water molecules from the plane and the sulfate group using hydrogen bonds.
Thermal Impact
When heated, the pentahydrate sequentially cleaves two water molecules, passing into the CuSO 4 · 3H 2 O trihydrate (this process, weathering , also proceeds slowly at lower temperatures [including at 20–25 ° С]), then to the monohydrate (at 110 ° C) CuSO 4 · H 2 O, and anhydrous salt forms above 258 ° C.
Above 650 ° C, the pyrolysis of anhydrous sulfate becomes intense according to the reaction:
Solubility
The solubility of copper (II) sulfate passes through a flat maximum as the temperature rises, during which the solubility of the salt remains almost unchanged (in the range of 80-200 ° C). (see fig.)
Like all salts formed by ions of a weak base and a strong acid, copper (II) sulfate hydrolyzes (the degree of hydrolysis in a 0.01 M solution at 15 ° C is 0.05%) and gives an acidic medium ( pH of this solution 4.2 ) The dissociation constant is 5–10 −3 .
Chemical Properties
Electrolytic Dissociation
CuSO 4 is a highly soluble salt in water and a strong electrolyte; in solutions of copper (II) sulfate, like all soluble salts, it dissociates in one stage:
Substitution Reaction
The substitution reaction is possible in aqueous solutions of copper sulfate using metals more actively than copper, standing to the left of copper in the electrochemical series of metal voltages:
Reaction with soluble bases (alkalis)
Copper (II) sulfate reacts with alkalis with the formation of a blue copper (II) hydroxide precipitate [7] :
Abbreviated Ion Equation ( Bertollet Rule )
Exchange with other salts
Copper sulfate also enters into exchange reactions for ions of Cu 2+ and SO 4 2-
Other
With sulfates of alkali metals and ammonium forms complex salts, for example, Na 2 [Cu (SO 4 ) 2 ] · 6H 2 O.
The Cu 2+ ion turns the flame green.
Production and Application
Copper (II) sulfate is one of the most important salts of copper. Often serves as feedstock for other copper compounds.
Anhydrous copper sulfate is a good dehumidifier and can be used to absolve ethanol , drain gases (including air) and as an indicator of humidity.
The ease of growing crystals of copper sulfate pentahydrate and their sharp difference with the anhydrous form are used in school education.
In construction, an aqueous solution of copper sulfate is used to neutralize the effects of leaks, eliminate rust stains, and also to remove salt (“efflorescence”) from brick, concrete and plaster surfaces, as well as an antiseptic and fungicidal agent to prevent rotting of wood.
In agriculture, copper sulfate is used as an antiseptic, fungicide and copper-sulfur fertilizer. To disinfect tree wounds, a 1% solution (100 g per 10 l) is used, which is rubbed into previously cleaned damaged areas. Against late blight of tomatoes and potatoes, plantings are sprayed with a 0.2% solution (20 g per 10 l) at the first signs of the disease, as well as for prevention in case of a threat of the disease (for example, in wet, humid weather). A solution of copper sulfate is watered to disinfect and make up for the lack of sulfur and copper (5 g per 10 l). However, more often, copper sulfate is used as part of Bordeaux fluid - the main copper sulfate CuSO 4 · 3Cu (OH) 2 against fungal diseases and grape phylloxera . For these purposes, copper (II) sulfate is commercially available.
To combat water flowering in reservoirs, chemical treatment with copper sulfate is also used [8] .
It is also used for the manufacture of mineral paints , in medicine, as one of the components of electrolytic baths for copper plating, etc., and as part of spinning solutions in the production of acetate fiber .
In the food industry is registered as a food additive E519 . It is used as a fixative for coloring and preservative.
In everyday life, it is used to remove rust stains on the ceiling after flooding.
In points of purchase of non-ferrous scrap metal, a solution of copper sulfate is used to detect zinc , manganese and magnesium in aluminum alloys and stainless steel . When these metals are detected, red spots appear.
Toxicology
Copper (II) sulfate is a compound with moderate toxicity and belongs to hazard class 4 (low-hazard substance). The lethal dose of copper sulfate is from 45 to 125 grams for an adult orally (if swallowed), depending on weight, state of health, immunity to excess copper and other factors. Signs of poisoning become noticeable with a single consumption of more than 0.5 g of the compound inside (the so-called toxic dose ). LD 50 for rats was 612.9 mg / kg [2] . The pattern of poisoning by inhalation of aerosols is more complex.
Skin contact with dry matter is safe, but must be washed off. Likewise in contact with solutions and wetted solids. In case of contact with eyes, rinse thoroughly with running water (light stream). If solid substance or concentrated solutions enter the gastrointestinal tract, it is necessary to rinse the victim’s stomach with a 0.1% potassium permanganate solution, give the patient a saline laxative — magnesium sulfate 1-2 tablespoons, induce vomiting, and give a diuretic. In addition, anhydrous substances in the mouth and stomach can cause thermal burns .
Weak copper sulfate solutions when taken orally act as a powerful emetic and are sometimes used to induce vomiting.
When working with powders and dust of copper (II) sulfate, care should be taken to prevent dusting, it is necessary to use a mask or respirator, and after work, wash your face. An acute toxic dose by inhalation of an aerosol is 11 mg / kg [9] . If copper sulfate enters the respiratory tract in the form of an aerosol, you need to remove the victim to fresh air, rinse your mouth with water and rinse the wings of the nose.
The substance should be stored in a cool, dry place, in tightly closed tight plastic or glass packaging, separately from medicines, food and animal feed, out of the reach of children and animals.
Notes
- ↑ Copper sulfate . kristallov.net. Date of appeal April 26, 2017.
- ↑ 1 2 Ershov Yu. A. , Pletneva T.V. Mechanisms of the toxic effect of inorganic compounds. - M .: Medicine, 1989 .-- S. 142.
- ↑ Copper sulfate // Chemical Encyclopedia / Ch. ed. I. L. Knunyants , N. S. Zefirov . - M .: Soviet Encyclopedia , 1990. - T. 3. - ISBN 5-85270-008-8 .
- ↑ 1 2 3 Karjakin Yu. V. Pure chemical reagents. Guidelines for the laboratory preparation of inorganic preparations. - 2nd ed. - M. — L .: GHI, 1947. - S. 343. - 577 p.
- ↑ Polyansky N.A., Kozhevnik S.N. Purification of copper compounds from impurities. Preparation of high purity copper sulfate // Collection of laboratory works. - Norilsk, 1998.
- ↑ Handbook of a chemist . - 2nd ed., Revised. and add. - L. — M .: Chemistry, 1963. - T. 2. - S. 124-125, 265. - 1168 p. - 20,000 copies.
- ↑ Obtaining insoluble bases . A single collection of the ZOR. Date of appeal April 26, 2017.
- ↑ Agricultural water supply and watering. - The ear. - M. , 1984.
- ↑ Copper Sulfate . Pesticide Management Education Program (PMEP) . Cornell University (December 1993). Date of appeal April 26, 2017.

